
����Ŀ���й����ʵ����ļ�������ѧ��ѧ����Ҫ���֣���ش������й����ʵ����ļ������⡣
��1���ڱ�״���£�67.2 L CO2��__________mol������Ϊ_______g������__________��CO2���ӣ����к���__________mol��ԭ�ӡ�
��2���ڱ�״���£�1.7 g������ռ�����ԼΪ_________L������ͬ������_____mol H2S������ͬ����ԭ������
��3��ij��̬�����ﻯѧʽΪRO2���ڱ�״���£�1.28 g��������������448 mL�����������Ħ������Ϊ_______��R�����ԭ������Ϊ__________��
��4��ʵ���ҳ���Ũ�������������Ϊ98%���ܶ�Ϊ1.80 g��mL1�������ʵ���Ũ����_______��
��5����״���£���V L A���壨Ħ������ΪM g/mol����ȫ����0.1 Lˮ���ܶ�1 g/cm3���У�������Һ���ܶ�Ϊd g/mL�������Һ�����ʵ���Ũ��Ϊ_______mol/L��
A��![]() B��
B��![]() C��
C��![]() D��
D��![]()
���𰸡�3.0 132 3NA 6 2.24 0.15 64 g/mol 32 18.4 mol/L B
��������
��1������n=V/Vm������̼�����ʵ���������m=nM����������������N=nNA���������Ŀ����ԭ�����ʵ���Ϊ������̼��2����
��2������n=m/M���㰱�����ʵ���������V=nVm���㰱�����������Hԭ����Ŀ��ȼ�����������ʵ�����
��3������n=V/Vm����������ʵ���������M=m/n�����������Ħ����������������R�����ԭ��������
��4������c=1000��w/M�����Ũ��������ʵ���Ũ�ȣ�
��5������n=V/22.4L��mol��1��������VL����������ʵ�����������m=nM�����������������ܼ������ʵ�������Ϊ��Һ��������Ȼ������V=m/�Ѽ�����Һ��������������c=n/V�������Һ�����ʵ���Ũ�ȣ�
��1��������̼�����ʵ���Ϊ67.2L/22.4L��mol��1=3mol��
������Ϊ3mol��44g��mol��1=132g��
������̼������ĿΪ3mol��6.02��1023mol��1=1.806��1024��
��ԭ�����ʵ���Ϊ3mol��2=6mol��
��2��1.7g �������ʵ���Ϊ1.7g/17g��mol��1=0.1mol���������Ϊ0.1mol��22.4L��mol��1=2.24L��
�뺬����ͬHԭ����Ŀ����������ʵ���Ϊ0.1mol��3/2=0.15mol��
��3������������ʵ���Ϊ0.448L/22.4L��mol��1=0.02mol���������Ħ������Ϊ1.28g/0.02mol=64g��mol��1��R�����ԭ������Ϊ64-32=32��
��4���ܶ�Ϊ1.84g��cm��3����������Ϊ 98% ��Ũ���ᣬ�����ʵ���Ũ��=1000��1.84��98%/98mol��L��1=18.4 mol��L��1��
��5����״���£���������ʵ���ΪVL/22.4L��mol��1=V/22.4mol�������������Ϊ��V/22.4mol��M g��mol��1=VM/22.4g��0.1Lˮ������Ϊ��100mL��1g��mL��1=100g������Һ������Ϊ��VM/22.4g+100g�����Ը���Һ�����Ϊ��![]() L��
L��
�����Һ�����ʵ���Ũ��Ϊ��c=n/V=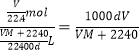 mol��L��1��
mol��L��1��
��ѡB��


 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ж�����������ȷ����
A.ֻ�����������вŴ���̼̼����B.���е���������ȼ��
C.����ͨʽΪCnH2n+2����������������Ҳ������ϩ��D.��ϩ���⣬������������ʹ����KMnO4��Һ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪���������������¼��֣�
�����Ӽ� �ڼ��Թ��ۼ� �۷Ǽ��Թ��ۼ� ����� �ݷ��Ӽ�������
������ijͬѧ��һЩ�仯�����ƻ������������õ��жϣ�
A���ɱ��ۻ��ڢ� B��������������ˮ��
C���Ȼ�����������ˮ �ڢ� D�����ۻ��ڢ�
�����ж���ȷѡ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ڷ�����ᴿ���ʵķ����У����ˡ������ᾧ��������ȡ����Һ��ϴ������������������װ�õ���Ų����ϲ������ƣ�
��1������Na2CO3��Һ��CCl4��ѡ_____����������Ϊ________��
��2����CCl4��ȡ��ˮ�еĵ⣬ѡ______����������Ϊ________��
��3��������������Һ����O2�л��е�����Cl2��ѡ_______����������Ϊ________��
��4����ȥ����ʯ��ˮ��������CaCO3����ѡ______����������Ϊ__________��
��5����ȡ����ˮ��ѡ________����������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
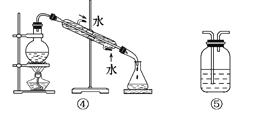
����Ŀ��ʵ��������Ҫ������NaCl��Һ��������ֻ�л���Na2SO4��NH4HCO3��NaCl��ijѧ���������ͼ��ʾ������ȡ������NaCl��Һ��
(��֪��NH4HCO3![]() NH3����CO2����H2O)
NH3����CO2����H2O)
����˷�����ȷ���ش��������⣺
��1�������ٿ�ѡ�����Ҫ������____________��____________��
��2�������ڲ������ᱵ��Һ����������__________________________________________��
��3�����в����ں�����ж�SO42 �ѳ�����������_________________________��
��4�������۵�Ŀ����________________��
��5�������ܵ�Ŀ����__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ�����С��������һ�ִӷϾɵĺ�������(��Ҫ�ɷ�ΪNiO������Fe2O3��CaO��CuO��BaO�ȣ����������¹��ա�������������ͼ��
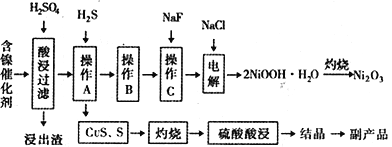
�ش�����������
��1����������Ҫ�ɷ�ΪCaSO4����2H2O��_______________�������ʡ�
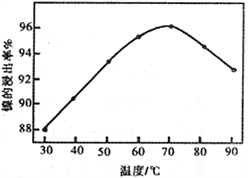
��2����ͼ��ʾ���Ľ��������¶ȵĹ�ϵ���������¶ȸ���70��ʱ�����Ľ����ʽ�������������Ni(OH)2����������ԭ����_____________________________��
��3�����������еġ�����Ʒ��Ϊ________���ѧʽ����
��4����֪�й��������↑ʼ�����ͳ�����ȫ��pH ���±���
�������� | Fe(OH)3 | Fe(OH)2 | Ni(OH)2 |
��ʼ������pH | 1.5 | 6.5 | 7.7 |
������ȫ��pH | 3.7 | 9.7 | 9.2 |
����B��Ϊ�˳�ȥ��Һ�е���Ԫ�أ�ijͬѧ���������ʵ�鷽���������A���õ���Һ�м���NaOH��Һ��������ҺpHΪ3.7��7.7�����ã����ˡ���Ը�ʵ�鷽���Ƿ���ȷ�����жϲ����������� ___________________________________����ԭ������ȷ����˵�����ɣ���ԭ������������Ը�������
��5������C��Ϊ�˳�ȥ��Һ�е�Ca2+����������Һ��F��Ũ��Ϊ3��10-3mol��L-1������Һ��![]() =________________��������ʱ��Ksp(CaF2)=2.7��10-11��
=________________��������ʱ��Ksp(CaF2)=2.7��10-11��
��6��������2NiOOH��H2O��ԭ����������
�ټ���������Cl-������������ΪClO-������1mol ClO-������OH-______________mol��
��Ni2+��ClO-��������2NiOOH��H2O��������ò���Ӧ�����ӷ���ʽΪ_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������¼�����Ӧ��
��Cl2��2KI===2KCl��I2 ��2FeCl2��Cl2===2FeCl3
��2FeCl3��2KI===2FeCl2��2KCl��I2
��I2��SO2��2H2O===H2SO4��2HI
�ж���������ǿ������˳����
A. Cl2��I2��Fe3����SO2 B. Cl2��Fe3����I2��SO2
C. Cl2��Fe3����SO2��I2 D. Fe3����I2��Cl2��SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȡ100 mL0.3mol/L��300 mL0.25mol/L������ע��500 mL������ƿ������ˮϡ�����̶�������û��Һ��H+�����ʵ���Ũ��Ϊ
A. 0.21mol/L B. 0.26mol/L C. 0.42mol/L D. 0.56mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
BԪ��ԭ�ӵĺ���p��������s��������1 |
Cԭ�ӵĵ�һ�����ĵ����ֱܷ��� I1��738 kJ/mol��I2��1 451 kJ/mol��I3��7 733 kJ/mol��I4��10 540 kJ/mol |
Dԭ�Ӻ�������p���ȫ������� |
EԪ�ص������������������IJ�Ϊ4 |
F��ǰ�������е縺����С��Ԫ�� |
G�����ڱ��ĵ����� |
��1����֪BA5Ϊ���ӻ����д�������ʽ______________________________��
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ���________������ԭ�ӹ����________�Ρ�
��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ![]() ��ͬѧ�����ĵ����Ų�ͼΥ����_____________________________________��
��ͬѧ�����ĵ����Ų�ͼΥ����_____________________________________��
��4��Gλ��________��________�����۵����Ų�ʽΪ________��
��5������FԪ�صķ�����________������ԭ�ӽṹ��֪ʶ���Ͳ����������ԭ����_____________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com