
����Ŀ��ʵ��������500mL0.1mol/LNa2CO3��Һ���ش���������
��1������Na2CO3��Һʱ���õ���Ҫ������������ƽ����ֽ���ձ���ҩ�ס�___��
��2������ƿ�ϱ��п̶��ߡ�___��ʹ��ǰҪ___��
��3������������ƽ��ȡNa2CO3____g��
��4����ʵ���������������Һ��Ũ����ƫ�ߣ�ƫ�ͻ��Dz��䣿
A.��ˮʱ�����̶���___��
B.�ܽ��δ��ȴ�����¾�ת������ƿ___��
C.����ƿ�ڱڸ���ˮ���δ���ﴦ��___��
D.����ʱ����___��
E.���µߵ�ҡ�Ⱥ�Һ����ڿ���___��
��5����ʵ������Ҫ��Ũ��Ϊ16mol/L��Ũ��������480mL2.0mol/L��ϡ���ᣬ����Ҫ��ȡŨ��������Ϊ___mL��
���𰸡�500mL����ƿ������������ͷ�ι� �¶ȡ��ݻ� ��© 5.3 ƫ�� ƫ�� ���� ƫ�� ���� 62.5
��������
����һ�����ʵ���Ũ����Һ�����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���������ʱ�ɸ���c=![]() �жϡ�
�жϡ�
(1)�������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ�����ҡ�ȡ����ݡ�ҡ�ȡ�װƿ��֪����������У�������ƽ���ձ�����������ҩ�ס�500mL����ƿ�ͽ�ͷ�ιܣ�ȱ�ٵ�������500mL����ƿ����ͷ�ιܡ���������
(2)����ƿ�ϱ����¶ȡ��̶��ߡ��ݻ�������ƿ����ƿ����Ϊ��ֹʹ�ù�����©Һ��ʹ��ǰӦ��©��
(3)����500mL0.1mol/LNa2CO3����ҪNa2CO3������Ϊ��0.5L��0.1mol/L��106g/mol=5.3g��
(4)A����ˮʱ�����̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ�
B���ܽ��δ��ȴ�����¾�ת������ƿ����ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ�
C������ƿ�ڱڸ���ˮ���δ���ﴦ�������������ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣻
D������ʱ���ӣ�������Һ���ƫ����ҺŨ��ƫ�ͣ�
E�����µߵ�ҡ�Ⱥ�Һ����ڿ��ߣ�����������������ҺŨ�Ȳ��䣻
(5)��ʵ������Ҫ��Ũ��Ϊ16mol/L��Ũ��������480mL2.0mol/L��ϡ���ᣬӦѡ��500mL����ƿ������ҪŨ�������ΪV����������Һϡ�������������ʵ�������ã�16mol/L��V=500mL2.0mol/L�����V=62.5mL��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�̶��ݻ�Ϊ2 L���ܱ������м���2 molA��3 molB�������¶�Ϊ30�棬�ڴ������ڵ������½������з�Ӧ��2A(g)+3B(g) ![]() 3C(g)��2���Ӵﵽƽ�⣬����1.5 mol C����ʱ��ƽ��������C���������Ϊ��1�������¶����ߵ�70����������������䣬����Ӧ���´ﵽƽ��ʱ��C�����ʵ���Ϊ2.1 mol���������Ϊ��2����ش��������⣬
3C(g)��2���Ӵﵽƽ�⣬����1.5 mol C����ʱ��ƽ��������C���������Ϊ��1�������¶����ߵ�70����������������䣬����Ӧ���´ﵽƽ��ʱ��C�����ʵ���Ϊ2.1 mol���������Ϊ��2����ش��������⣬
��1���÷�Ӧ��30��ʱƽ�ⳣ��K1=_________ ���ʱ��H_______0(�>������<����=��)��
��2���ӷ�Ӧ��ʼ���ﵽ��ѧƽ��״̬v(A)____________mol/(L��min)
��3���÷�Ӧ��70��ʱƽ�ⳣ��ΪK2���� K1_______K2(�����������������)
��4�������ж�2A(g)+3B(g) ![]() 3C(g) ��Ӧ�Ѿ��ﵽƽ����____��
3C(g) ��Ӧ�Ѿ��ﵽƽ����____��
A��2v(B)��3v(A)
B���ܱ���������ѹǿ����
C. �ܱ������л��������ܶȲ���
D����������ƽ����Է����������ٸı�
E��n(A)��n(B)��n(C)��2��3��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������Һ���й���������ȷ����
��� | �� | �� | �� | �� |
pH | 11 | 11 | 3 | 3 |
��Һ | ��ˮ | ����������Һ | ���� | ���� |
A. �ۢ��зֱ���������Ĵ����ƾ��������Һ��pH������
B. �ڢ�����Һ�������ϣ�������Һ��c��H+��>c��OH-��
C. �ֱ��ˮϡ��10����������Һ��pH����>��>��>��
D. �������Һ�У�ˮ�ĵ���̶ȣ���>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪ��ʯ����ȡ���͵�װ��ʾ��ͼ������ͼʾ�ش��������⡣

(1)ͼ�е��������ԵĴ�����________________��__________________
(2)A������������________��B������������________��
(3)ʵ��ʱ A �г�����ʯ���⣬�����������__________����������__________________��
(4)�ռ������ͺ����ȳ��ƾ��ƻ�����ͣ����ˮ��
______________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ⶨˮ���ܽ����ķ����ǣ�
����ȡa mLˮ����Ѹ�ټ���̶���MnSO4��Һ�ͼ���KI��Һ(��KOH)����������ƿ����������ʹ֮��ַ�Ӧ���䷴ӦʽΪ��2Mn2����O2��4OH��===2MnO(OH)2(�÷�Ӧ����)��
�ڲⶨ��������Ѹ�ټ���1��2 mLŨ����(�ữ���ṩH��)��ʹ֮����I2������b mol/L��Na2S2O3��Һ�ζ�(�Ե���Ϊָʾ��)������V mL���йط�ӦʽΪ��MnO(OH)2��2I����4H��===Mn2����I2��3H2O��I2��2S2O32-===2I����S4O62-��
�Իش�
��1���ζ�����ʱ�����ֿ��Ƶζ��ܣ�����_________________���۾�Ҫע��__________________________��
��2���ζ�(I2��S2O32-��Ӧ)�Ե���Ϊָʾ�����յ�ʱ��Һ��ɫ�仯Ϊ_________________��
��3��ˮ���ܽ����ļ���ʽ��_______________(��g/LΪ��λ)��
��4���ⶨʱ���ζ��ܾ�����ˮϴ�Ӻӵζ���Na2S2O3��Һ�����²ⶨ���________(����ƫ������ƫ����������Ӱ��������ͬ)��
��5����¼�ⶨ���ʱ���ζ�ǰ���ӿ̶��ߣ��ζ������յ�ʱ�ָ��ӿ̶��ߣ������µζ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܱ������У���ӦX(g)+2Y(g)![]() Z(g)�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc(X)=0.100mol��L��1��c(Y)=0.200mol��L��1��c(Z)=0mol��L��1����Ӧ��X��Ũ����ʱ��ı仯��ͼ��ʾ������˵����ȷ����
Z(g)�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc(X)=0.100mol��L��1��c(Y)=0.200mol��L��1��c(Z)=0mol��L��1����Ӧ��X��Ũ����ʱ��ı仯��ͼ��ʾ������˵����ȷ����
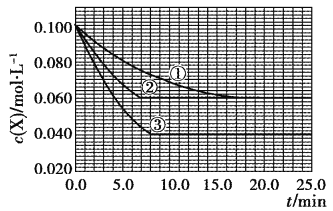
A. ʵ��ڵ��¶ȸ���ʵ��ٵ��¶�
B. ʵ���ƽ��ʱY��ת����Ϊ60%
C. ʵ�����ʵ�����ȣ�ʵ���ʹ���˴���
D. ��ӦX(g)+2Y(g)![]() Z(g)�ġ�H>0
Z(g)�ġ�H>0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ʵ���Ũ�ȵ����ᡢ����������Һ�ֱ���ڼס������ձ��У���������������������������������Ϊ5:6����ס������ձ��еķ�Ӧ������ֱܷ���
A. �ס����ж��������� B. ���������������м����
C. ��������������������� D. �����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������H��He��N��Na��Mg��Si��Ԫ�أ�������δ������Դ���⡣
��1��3He�Ǹ�Ч����ԭ�ϣ���ԭ�Ӻ���������Ϊ_____________��
��2��Na��ԭ�ӽṹʾ��ͼΪ______��Na����������ȫȼ�����ò���ĵ���ʽΪ_______��
��3��MgCl2�ڹ�ҵ��Ӧ�ù㷺������MgO�Ʊ���
��MgO���۵��BaO���۵�________��������������������
��������ij��ʯ�������õ���MgO�к�������SiO2����ȥSiO2�����ӷ���ʽΪ______��SiO2�ľ�������Ϊ________��
��MgO��̿�ۺ�������һ�������·�Ӧ���Ʊ�MgCl2����β����������NaOH��Һ��ȫ���գ������ɵ���Ϊ______��д��ѧʽ����
��4�������к��зḻ��3He��������������1 kg3Heͬʱ�ɵ�6000kgH2��700kgN2����������H2��N2Ϊԭ�Ͼ�һϵ�з�Ӧ�����Ƶ�̼�����___kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�����ֵ�������й�������ȷ����(����)
A. 14 g��ϩ�ͱ�ϩ��������е���ԭ����Ϊ2NA
B. 1 mol N2��4 mol H2��Ӧ���ɵ�NH3������Ϊ2NA
C. 1 mol Fe���ڹ������ᣬ����ת����Ϊ2NA
D. ��״���£�2.24 L CCl4���еĹ��ۼ���Ϊ0.4NA
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com