
����Ŀ���������ⶨˮ���ܽ����ķ����ǣ�
����ȡa mLˮ����Ѹ�ټ���̶���MnSO4��Һ�ͼ���KI��Һ(��KOH)����������ƿ����������ʹ֮��ַ�Ӧ���䷴ӦʽΪ��2Mn2����O2��4OH��===2MnO(OH)2(�÷�Ӧ����)��
�ڲⶨ��������Ѹ�ټ���1��2 mLŨ����(�ữ���ṩH��)��ʹ֮����I2������b mol/L��Na2S2O3��Һ�ζ�(�Ե���Ϊָʾ��)������V mL���йط�ӦʽΪ��MnO(OH)2��2I����4H��===Mn2����I2��3H2O��I2��2S2O32-===2I����S4O62-��
�Իش�
��1���ζ�����ʱ�����ֿ��Ƶζ��ܣ�����_________________���۾�Ҫע��__________________________��
��2���ζ�(I2��S2O32-��Ӧ)�Ե���Ϊָʾ�����յ�ʱ��Һ��ɫ�仯Ϊ_________________��
��3��ˮ���ܽ����ļ���ʽ��_______________(��g/LΪ��λ)��
��4���ⶨʱ���ζ��ܾ�����ˮϴ�Ӻӵζ���Na2S2O3��Һ�����²ⶨ���________(����ƫ������ƫ����������Ӱ��������ͬ)��
��5����¼�ⶨ���ʱ���ζ�ǰ���ӿ̶��ߣ��ζ������յ�ʱ�ָ��ӿ̶��ߣ������µζ����________��
���𰸡���������ƿ ��ƿ����Һ��ɫ�ı仯 ����ɫ��Ϊ��ɫ 8bV/a ƫ�� ƫ��
��������
��1���ζ�ʱ�����ֿ��Ƶζ��ܻ����������ճ���ƿ���ߵα����۾�ע����ƿ����Һ��ɫ�ı仯��
��2������I2�ĵ�����Һ����ɫ������S2O32-����I2���յ�ʱI2��ȫ��Ӧ��
��3�����ݹ�ϵʽ��O2��2MnO��OH��2��2I2��4S2O32-�ɼ���ˮ�����ܽ�����Ũ�ȣ�
��4�������ܽ���Ũ�ȱ���ʽ��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��5�������ܽ���Ũ�ȱ���ʽ��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��1���ζ�����ʱ�����ֿ��Ƶζ��ܣ����ֲ�������ƿ���۾�Ҫע����ƿ����Һ��ɫ�ı仯��
��2������I2�ĵ�����Һ����ɫ������S2O32-����I2���յ�ʱI2��ȫ��Ӧ���յ�ʱ��Һ����ɫɫ��Ϊ��ɫɫ���Ұ�����ڲ���ɫ��
��3�����ݷ�Ӧ��2Mn2++O2+4OH-�T2MnO��OH��2�� MnO��OH��2+2I-+4H+�TMn2++I2+3H2O��I2+2S2O32-�TS4O62-+2I-��
��֪��ϵʽ��O2��2MnO��OH��2��2I2��4S2O32-��
32g 4mol
m bmol/L![]() VmL
VmL![]() 10-3L/mL
10-3L/mL
���m=8bV��10-3g����1Lˮ������������Ϊ��![]() g/L��
g/L��
��4���ⶨʱ���ζ��ܾ�����ˮϴ�Ӻӵζ���Na2S2O3��Һ����Һ�屻ϡ�ͣ�Ũ�ȱ�ϡ���Vƫ�����ܽ���![]() g/L����֪Ũ��ƫ�ߣ�
g/L����֪Ũ��ƫ�ߣ�
��5����¼�ⶨ���ʱ���ζ�ǰ���ӿ̶��ߣ�����ƫ�ζ������յ�ʱ�ָ��ӿ̶��ߣ�����ƫС�����VƫС�������ܽ���![]() g/L����֪Ũ��ƫ�͡�
g/L����֪Ũ��ƫ�͡�


 ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��һ���¶��£��������ˮϡ��������Һ�ĵ�����������ͼ����ش�

��1����O����Ϊʲô������___________________��
��2��a��b��c�����������Ũ����С�����˳��Ϊ____________��
��3��a��b��c�����У�����ĵ���̶�����һ����_________��
��4����ʹc����Һ�е�c(CH3COO��)��ߣ������´�ʩ�У���ѡ��__________��
A������ B���Ӻ�ϡ��NaOH��Һ C���ӹ���KOH D����ˮ
E���ӹ���CH3COONa F����Zn�� G����MgO���� H����Na2CO3����
��5����ϡ�����У����Ŵ���Ũ�ȵĽ��ͣ�����ʼ�ձ����������Ƶ�����______________��
A��c(H��) B��H������ C��CH3COOH������ D��c(H��)/c(CH3COOH)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ģ������ģ�8�ǻ�������㷺�����������ӵ���ϼ�����ȡ����Ҳ����Ҫ��ҽҩ�м��塣��ͼ��8�ǻ�����ĺϳ�·�ߡ�

��֪��i. 
ii.ͬһ��̼ԭ��������2���ǻ��ķ��Ӳ��ȶ���
��1���������ŷ��࣬A�������__________��
��2��A��B�Ļ�ѧ����ʽ��____________________��
��3��C���ܵĽṹ��ʽ��__________��
��4��C��D������Լ�a��__________��
��5��D��E�Ļ�ѧ����ʽ��__________��
��6��F��G�ķ�Ӧ������__________��
��7��������K��L������ͼ����������____________

��8���ϳ�8�ǻ����ʱ��L������__________�����������ԭ������Ӧ����Ӧʱ��������ˮ����L��G���ʵ���֮��Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ���ĸ�ͼ���˵����ȷ����


ע��ͼ�У�E��ʾ������p��ʾѹǿ��t��ʾʱ�䣬V��ʾ�����
A. ����ʾ��ѧ��ӦH2(g)��Cl2(g)===2HCl(g)�������仯����÷�Ӧ�ķ�Ӧ����H��+183 kJ/mol
B. ����ʾ������������ʱ����Ӧ4A(g)��3B(g)![]() 2C(g)��6D�ڲ�ͬѹǿ��B�����������ʱ��ı仯����Dһ��������
2C(g)��6D�ڲ�ͬѹǿ��B�����������ʱ��ı仯����Dһ��������
C. ����ʾ�����pH����ͬ��HCl��CH3COOH������Һ�У��ֱ����������п������H2�������ʱ��ı仯����a��ʾCH3COOH��Һ
D. ����ʾ10 mL 0.1 mol/L Na2CO3��NaHCO3������Һ�У��ֱ�μ�0.1 mol/L���ᣬ����CO2���������������ı仯����b��ʾNa2CO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʹ�õ�����CuSO45H2O������0.1 molL-1������ͭ��Һ����ȷ�IJ����ǣ� ��
A. ��ȡ25 g�������ܽ���1 Lˮ��
B. ��ȡ25 g��������ˮ��Ȼ����Һϡ����1L
C. ��16 g��������ˮ��Ȼ����Һϡ����1L
D. ���������ȳ�ȥ�ᾧˮ��ȡ16 g�ܽ���1Lˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ��������500mL0.1mol/LNa2CO3��Һ���ش���������
��1������Na2CO3��Һʱ���õ���Ҫ������������ƽ����ֽ���ձ���ҩ�ס�___��
��2������ƿ�ϱ��п̶��ߡ�___��ʹ��ǰҪ___��
��3������������ƽ��ȡNa2CO3____g��
��4����ʵ���������������Һ��Ũ����ƫ�ߣ�ƫ�ͻ��Dz��䣿
A.��ˮʱ�����̶���___��
B.�ܽ��δ��ȴ�����¾�ת������ƿ___��
C.����ƿ�ڱڸ���ˮ���δ���ﴦ��___��
D.����ʱ����___��
E.���µߵ�ҡ�Ⱥ�Һ����ڿ���___��
��5����ʵ������Ҫ��Ũ��Ϊ16mol/L��Ũ��������480mL2.0mol/L��ϡ���ᣬ����Ҫ��ȡŨ��������Ϊ___mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijѧ����������ͼװ�ã���ƿλ�ò����ƶ����ռ��������壺
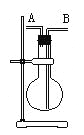
�� H2��Cl2��CH4��HCl ��NH3��NO ��NO2��SO2��
���в�����ȷ����
A. ��ƿ�Ǹ���ģ���A�����ռ��٢ۢ�
B. ��ƿ�Ǹ���ģ���B�����ռ��ڢܢޢߢ�
C. ����ƿ�г���ˮ����A�����ռ��٢ۢݢ�
D. ����ƿ�г���ˮ����B�����ռ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Դ�����ͬ��ע����Ҫ���⣬������һ�ֽྻ����Դ��
(1)���鲻��������ȼ�ϣ��������������ϳ���(CO��H2)��CH4��H2O(g)ͨ��۽�̫���ܷ�Ӧ����������ӦCH4(g)+H2O(g)=CO(g)+3H2(g)��H1����֪ijЩ��ѧ���ļ����������±���
��ѧ�� | C��H | H��H | C��O | O��H |
����/kJ��mol��1 | 413 | 436 | 1076 | 463 |
���H1=___________kJ��mol��1
(2)�úϳ������ɼ״��ķ�ӦΪ��CO(g)+2H2(g)![]() CH3OH(g)��H2����2L�����ܱ������У������ʵ���֮��1��2����CO��H2�����CO��ƽ��ת�������¶Ⱥ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CH3OH(g)��H2����2L�����ܱ������У������ʵ���֮��1��2����CO��H2�����CO��ƽ��ת�������¶Ⱥ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
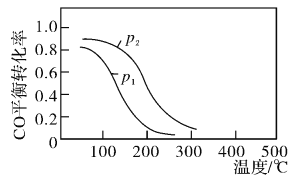
200��ʱn(H2)��ʱ��ı仯���±���ʾ
t/min | 0 | 1 | 2 | 3 |
n(H2)/mol | 6.0 | 3.4 | 2.0 | 2.0 |
�١�H2___________0(�>����<����=��)
������˵����ȷ����___________(����ĸ���)��
a.��ƽ����������г���ϡ�����壬ѹǿ����ƽ��������Ӧ�����ƶ�
b.�����¶ȣ��÷�Ӧ��ƽ�ⳣ�����
C.�����������ܶȲ��䣬��Ӧ�ﵽ�����
d.ͼ��ѹǿp1>p2
��200��ʱ���÷�Ӧ��ƽ�ⳣ��K=___________��
(3)���顢������KOH��Һ�����ȼ�ϵ�ء����ȼ�ϵ�ع���ʱ��ͨ�����ĵ缫Ϊ___________������缫��ӦʽΪ______________________��ͨ�������ĵ缫��ӦʽΪ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��298 K��101kPaʱ��N2��H2��Ӧ�����������仯������ͼ�����������������(����)
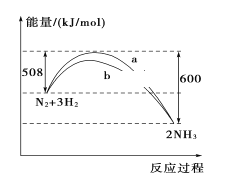
A. ������������ܸı�û�ѧ��Ӧ�ķ�Ӧ��
B. b�����Ǽ������ʱ�������仯����
C. �÷�Ӧ���Ȼ�ѧ����ʽΪ��N2(g)��3H2(g)![]() 2NH3(g)����H����92 kJ/mol
2NH3(g)����H����92 kJ/mol
D. �ڳ��¡����һ���������£�ͨ��1 mol N2��3 mol H2����Ӧ��ų�������Ϊ92 kJ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com