
����Ŀ������������һ�����͵Ķ����������Ʒ������������ɫ��ɫ��ĩ���������ᣬ������ˮ���Ҵ����ܼ���ijʵ��С���ڵ��º�ͨ�백���ļ��������£����ø�����������ⷴӦ��ȡCaO2��8H2O����(�÷�Ӧ��һ�����ȷ�Ӧ)��
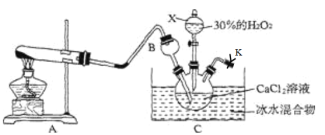
��1������X��������___���������װ�������Եķ���Ϊ��____
��2������B��������_______��CaO2�ĵ���ʽΪ______
��3��д��A�з�����Ӧ�Ļ�ѧ����ʽ��______��
��4����ȡCaO2��8H2Oʱ����ˮԡά�ַ�Ӧ��0��5��ĵ����½��У�ԭ����____������CaO2��8H2O�Ļ�ѧ����Ϊ____��
��5��2.76 gCaO2��8H2O��Ʒ������ˮ���̵���������(��Ʒ�������¶ȱ仯�����ߣ�140��ʱ��ȫ��ˮ���������Ȳ��ֽ�)��ͼ��ʾ��
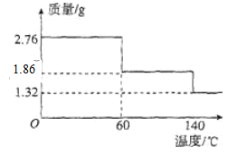
����ȷ��60��ʱCaO2��xH2O��x=______��
�ڸ���Ʒ��CaO2����������Ϊ______������4λ��Ч���֣���
���𰸡���Һ©�� ��K�رգ��ӷ�Һ©���м�ˮ��һ��ʱ���Һ�岻��˳������ ![]() ��ֹ���� 2NH4Cl��Ca(OH)2
��ֹ���� 2NH4Cl��Ca(OH)2![]() CaCl2��2NH3����2H2O ����0�棬Һ�����ᣬ��Ӧ���ѣ��¶ȹ��ߣ���������ֽ����ʼӿ� CaCl2��2NH3��8H2O+2H2O2=CaO2��8H2O+2NH4Cl 3 26.09��
CaCl2��2NH3����2H2O ����0�棬Һ�����ᣬ��Ӧ���ѣ��¶ȹ��ߣ���������ֽ����ʼӿ� CaCl2��2NH3��8H2O+2H2O2=CaO2��8H2O+2NH4Cl 3 26.09��
��������
��1������X��������֪Ϊ��Һ©�����������װ�������Եķ���Ϊ��K�رգ��ӷ�Һ©���м�ˮ��һ��ʱ���Һ�岻��˳�����£�˵�����������ã�
��2��������������ˮ������©������������ƿ����Һ�з�ֹ������CaO2Ϊ���ӻ�����ɸ����Ӻ�����������ɣ�
��3��ʵ���������Ȼ�狀��������ƹ��干����ȡ������
��4������O�棬Һ�����ᣬ��Ӧ���ѣ��¶ȹ��ߣ���������ֽ����ʼӿ죬CaCl2��H2O2��NH3��H2O��Ӧ����CaO28H2O��NH4Cl��
��5����140��ʱ��ȫ��ˮ���������Ȳ��ֽ⣬�����Ʒ������������ᾧˮ�������������ʵ������Ӷ��ó���Ʒ��CaO28H2O���ʵ������ٸ���60��ʱ��������������ʧȥ�ᾧˮ�����������ʵ��������������CaO2xH2O�е�x��
�ڸ��ݢټ��������Ʒ��CaO2�����ʵ���������m=nM��������������ټ������Ʒ��CaO28H2O�Ĵ���
��1������X�Ĺ����֪XΪ��Һ©�����������װ�������Եķ���Ϊ��K�رգ��ӷ�Һ©���м�ˮ��һ��ʱ���Һ�岻��˳�����£�˵�����������ã��ʴ�Ϊ����Һ©������K�رգ��ӷ�Һ©���м�ˮ��һ��ʱ���Һ�岻��˳�����£�
��2��BΪ����©��������������ƿ����Һ�У����Է�ֹ������������ˮ������������CaO2Ϊ���ӻ�����ɸ����Ӻ�����������ɣ�����ʽΪ![]() ���ʴ�Ϊ����������
���ʴ�Ϊ����������![]() ��
��
��3��ʵ���������Ȼ�狀��������ƹ��干����ȡ��������Ӧ�Ļ�ѧ����ʽΪ2NH4Cl��Ca(OH)2![]() CaCl2��2NH3����2H2O���ʴ�Ϊ��2NH4Cl��Ca(OH)2
CaCl2��2NH3����2H2O���ʴ�Ϊ��2NH4Cl��Ca(OH)2![]() CaCl2��2NH3����2H2O��
CaCl2��2NH3����2H2O��
��4�����ڵ���0�棬Һ�����ᣬ��Ӧ���ѣ����¶Ƚϸߣ���������ֽ����ʼӿ죬������ȡCaO28H2Oһ����0�桫5��ĵ����½��У�����װ��C�е����ʵó���ȡ��ԭ������ʽΪ��CaCl2+H2O2+2NH3+8H2O=2NH4Cl+CaO28H2O���ʴ�Ϊ������0�棬Һ�����ᣬ��Ӧ���ѣ��¶Ƚϸߣ���������ֽ����ʼӿ죻CaCl2+H2O2+2NH3+8H2O=2NH4Cl+CaO28H2O����5����140��ʱ��ȫ��ˮ���������Ȳ��ֽ⣬����Ʒ��CaO28H2O���еĽᾧˮ��������Ϊ��2.76g-1.32g��=1.44g���ᾧˮ�����ʵ���Ϊ![]() =0.08mol��60��ʱ���������Ϊ1.86g��ʧȥ�ᾧˮ������Ϊ��2.76g-1. 86g=0.9g��ʧȥ�ᾧˮ�����ʵ���Ϊ
=0.08mol��60��ʱ���������Ϊ1.86g��ʧȥ�ᾧˮ������Ϊ��2.76g-1. 86g=0.9g��ʧȥ�ᾧˮ�����ʵ���Ϊ![]() =0.05mol��ԭ��Ʒ�к���CaO28H2O�����ʵ���Ϊ
=0.05mol��ԭ��Ʒ�к���CaO28H2O�����ʵ���Ϊ![]() =0.01mol������60��ʱʧȥ�ᾧˮ�ĸ���Ϊ
=0.01mol������60��ʱʧȥ�ᾧˮ�ĸ���Ϊ![]() =5����60��ʱCaO2xH2O��x=8-,5=3���ʴ�Ϊ��3��
=5����60��ʱCaO2xH2O��x=8-,5=3���ʴ�Ϊ��3��
�ڸ��ݢٿ�֪CaO28H2O�����ʵ���Ϊ0.01mol����CaO2�����ʵ���Ҳ��0.01mol��������Ϊ��72g/mol��0.01mol=0.72g����Ʒ��CaO2�Ĵ���Ϊ![]() ��100%��26.09%���ʴ�Ϊ��26.09%��
��100%��26.09%���ʴ�Ϊ��26.09%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.̼Ԫ�ع㷺��������Ȼ���У����������������������Ҫ���塣��̼�������ڹ�ҵ���������������й㷺��Ӧ�á�
��1��д����̬̼ԭ�ӵĵ����Ų�ʽ________________�� CO2��_______���ӣ����������������Ǽ�������������̼ԭ�Ӳ�ȡ���ӻ�������_____________��
��2��̼����ͬ����Ԫ�أ�������˵�����߷ǽ��������ǿ������_________�����ţ���
a.CH4���ȶ��Ա�SiH4ǿ b.SiH4�ķе��CH4��
c.̼������Դ��ڹ��� d.SiO2+Na2CO3 =Na2SiO3+CO2��
II.�����裨Si3N4����һ����Ҫ���մɲ��ϣ�����ʯӢ�뽹̿��800�����������ºϳɣ�3SiO2(s)+6C(s)+2N2(g)![]() Si3N4(s)+6CO(g)
Si3N4(s)+6CO(g)
��1���÷�Ӧ��ƽ�ⳣ������ʽK=__________________����֪ƽ�ⳣ����K(800��)>K(850��)��������Ӧ��__________��Ӧ������������������������
��2����д��������߶�������ת���ʵĴ�ʩ______________��______________��
��3��Mg�ڿ�����ȼ�տ�����������þ��Mg3N2����Mg3N2����������ϡ����ɵõ��������Σ�д���÷�Ӧ�Ļ�ѧ����ʽ__________________________________________________��
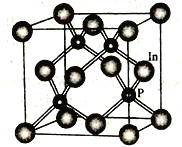
��4��������һ�ְ뵼����ϣ��侧������ͼ��ʾ��д�������Ļ�ѧʽ______________��In����λ������֮���������ԭ����Ŀ��Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ж���������Ԫ��R��X��Y��Z�������ǵ�������������n��ʾ�����У�n(X)��n(Y)��n(Z)��n(X)��n(Z)��n(R)��������Ԫ�����һ�ֻ�����Q��Q�����������ʣ�
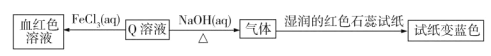
����˵���������
A.ԭ�Ӱ뾶��Y��Z��XB.����������Ӧˮ�������ԣ�Y��Z
C.X��Y��ɵĻ������ڳ����¶�����̬D.Y3Z4�ǹ��ۻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����������е㣺117. 2��������ȩ�е㣺75. 7������CH3CH2CH2CH2OH![]() CH3CH2CH2CHO��������ͼװ�����������ϳ�����ȩ������˵������ȷ����
CH3CH2CH2CHO��������ͼװ�����������ϳ�����ȩ������˵������ȷ����

A.Ϊ��ֹ�����һ��������Ӧ������Na2Cr2O7������Һ��μ�����������
B.���¶ȼ�1ʾ��Ϊ90~95�����¶ȼ�2ʾ����76������ʱ�ռ�����
C.��������������м������������ƣ��ɼ��������Ƿ���������
D.��������õĴ�����ȩ�У�����CaCl2���壬���ˣ������ᴿ����ȩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ô��м����ⶨ��Ȫʵ���е�ѹǿ�仯����ʶ��Ȫʵ���ԭ����ʵ��ʱ�ر�ͼ1�е�a���õ�����(��������ˮ�Ľ�ͷ�ι�)��������c����ˮ����������ƿ�У���b�������Ȫʵ�顣���Ի���������ƿ��ѹǿ�仯����ͼ2������˵����ȷ����
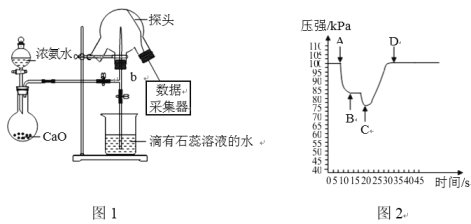
A.���ﰱ��ͨ����ѡ��Ũ����B.������ƿ�ڿ��Կ�����ɫ��Ȫ
C.��ͼ2��֪C��ʱ��Ȫ�����D.ͼ1�������ƿ����Ȼ��ƴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������������������������һ�ʻ������г�������������Ϊ���ӳ��ʻ���������ͨ��������ʻ����ʼ���
�±���0.25 Lij���ʻ����ʼ��к��еijɷּ�����(���ֳɷ�ʡ��)���ش��������⣺
�ɷ� | ����(g) | Ħ������(g/mol) |
����(C12H22O11) | 12.50 | 342 |
�����(K2SO4) | 0.125 | 174 |
�������(KMnO4) | 0.125 | 158 |
������(AgNO3) | 0.01 | 170 |
���� | ���� | ���� |
(1)�ʻ����ʼ������гɷ��У������ڵ���ʵ���__________(����)��
a��C12H22O11 b��K2SO4 c��KMnO4 d��AgNO3
(2)������250 mL���ʻ����ʼ��������ṩ������������250mL����ƿ����Ͳ���ձ���ҩ�עݵ�����ƽ����Ҫ���ʵ�飬ȱ�ٵIJ�����������_________��_________ (д��������)��
(3)���в�����ʹ�����ʻ����ʼ�Ũ��ƫ�͵���__________(����)��
a������ƿ������ˮϴ����û�к�� b���ò���������������Һת�Ƶ�����ƿ��ʱ����Һ����������ƿ���� c������ʱ���ӿ̶��� d���μ�����ˮ��ʹ��Һ����պ���̶������У�����ƿ������ҡ�Ⱥ��ã�����Һ��ȿ̶��ߵͣ��ټ�ˮ���̶���
(4)д�����ʻ����ʼ���K�������ʵ���Ũ�ȵļ���ʽ(ʡ�Գɷ��в���K��)_______(���ػ���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������(CaO2)��һ�ְ�ɫ���壬�ܳ��⣬������ˮ������ˮ������Ӧ���������Ҵ��������ᷴӦ��������ɱ�������������ȡ��������⣬�ش�������⡣
I��CaO2������Ʊ���
CaO2����ͨ��������CaCl2�ڼ�����������H2O2��Ӧ�Ƶá�ij��ѧ��ȤС����ʵ�����Ʊ�CaO2��ʵ�鷽����װ��ʾ��ͼ���£�

��1��������ƿ�з�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ_____��
��2����ˮԡ��Ŀ����____����������ϴ��CaO2��8H2O��ʵ�����������______
����CaO2�����IJⶨ��
�ⶨCaO2��Ʒ���ȵķ����ǣ���ȡ0.200g��Ʒ����ƿ�У�����50mLˮ��15mL2mol��L-lHCl����ʹ��Ʒ�ܽ����ɹ������⣬�ټ��뼸��MnCl2ϡ��Һ��������0.0200mol��L-lKMnO4����Һ�ζ����յ㣬����25.00mL��Һ��
��3������������ʹ��ϡ�������ʹ��ϡ�����ܽ���Ʒ��ԭ����___ ���ζ�ǰ����MnCl2ϡ��Һ�����ÿ�����____��
��4���ζ������е����ӷ���ʽΪ_______����Ʒ��CaO2����������Ϊ______ ��
��5��ʵ��I�Ƶõľ�����Ʒ��CaO2����ƫ�͵Ŀ���ԭ���ǣ���____���� ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ȼҵ�Ǹߺ��ܲ�ҵ,һ�ֽ�������ȼ�ϵ������ϵ��¹��տ��Խ���30%����,����ԭ����ͼ��ʾ,���и��缫δ����������й�˵��������ǣ� ��
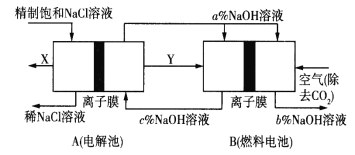
A.A�����ұ���NaOH��Һ��Ŀ����������Һ�ĵ�����
B.���ع���ʱ�ռ�����״��������XΪ2.24L,�������ϴ�ʱ�����״���µĿ���(������ȥ��CO2������仯)�����ԼΪ5.6L
C.AΪ�����ӽ���Ĥ��BΪ�����ӽ���Ĥ
D.�������Ƶ����������Ӵ�С��˳��Ϊb%>a%>c%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и���Һ�У�Na+���ʵ���Ũ�������ǣ�������
A. 4 L��0.5 mol��L-1NaCl��Һ B. 1 L��0.3 mol��L-1Na2SO4��Һ
C. 5 L��0.4 mol��L-1NaOH��Һ D. 2 L��0.15 mol��L-1��Na3PO4��Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com