
| �����Ϣ | |
| X | ����Ϊ˫ԭ�ӷ��ӣ��ڿ�������ռ���ԼΪ78% |
| Y | ��YԪ�ص�������ɫ��ӦΪ��ɫ |
| Z | ͬ����Ԫ����ԭ�Ӱ뾶��С |
| R | RԪ��ԭ�ӵ�������������K���������3�� |
 ��
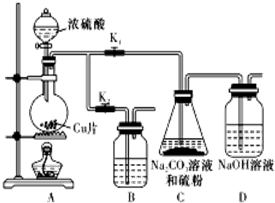
�� ��д������Һ�ڿ����б�������Y2R2�Ļ�ѧ����ʽ4Na2S+O2+2H2O�T4NaOH+2Na2S2��
��д������Һ�ڿ����б�������Y2R2�Ļ�ѧ����ʽ4Na2S+O2+2H2O�T4NaOH+2Na2S2������ I��X��Y��Z��R����Ԫ�ؾ�Ϊ������Ԫ�أ�X����Ϊ˫ԭ�ӷ��ӣ��ڿ�������ռ���ԼΪ78%����XΪNԪ�أ���YԪ�ص�������ɫ��ӦΪ��ɫ����YΪNa��Z��ͬ����Ԫ����ԭ�Ӱ뾶��С����Y��Z��Rͬ���ڣ���ZΪCl��RԪ��ԭ�ӵ�������������K���������3������RΪSԪ�أ�
��XΪNԪ�أ�Ԫ��X������γɶ��ֻ��������������죬����������ƣ�ΪһԪ���ᣬ����ķ���ʽΪHNm��HNm�ֽ�ķ���ʽΪ2HNm=H2+mN2��8.6gHNm��ը�ֽ�����H2��6.72L������£�N2������Ϊ$\frac{6.72L}{22.4L/mol}$=0.3mol����2��m=$\frac{8.6}{1+14m}$��0.3�����m=3�����Ա�ΪHN3����Ϊ���ӻ������ˮ��Ӧ����H2�������죬���������ܶ�Ϊ0.76g•L-1���������Է�������Ϊ0.76��22.4=17��������Ϊ��������ΪNH4H��
��Fe3+����Һ�зֲ�ˮ�⣬��һ��ˮ��̶����ˮ��̶����μ�С��
��� �⣺I��X��Y��Z��R����Ԫ�ؾ�Ϊ������Ԫ�أ�X����Ϊ˫ԭ�ӷ��ӣ��ڿ�������ռ���ԼΪ78%����XΪNԪ�أ���YԪ�ص�������ɫ��ӦΪ��ɫ����YΪNa��Z��ͬ����Ԫ����ԭ�Ӱ뾶��С����Y��Z��Rͬ���ڣ���ZΪCl��RԪ��ԭ�ӵ�������������K���������3������RΪSԪ�أ�
��1��ZΪCl��ZԪ�������ڱ���λ���ǵ������ڵڢ�A�壬���ӵ��Ӳ���Խ�࣬���Ӱ뾶Խ���Ӳ�ṹ��ͬʱ���˵����Խ�࣬���Ӱ뾶ԽС������Y��Z��R�����ӵİ뾶�Ӵ�С��˳���� S2-��Cl-��Na+��
�ʴ�Ϊ���������ڵڢ�A�壻 S2-��Cl-��Na+��
��2����N��Cl����Ԫ����ɵĻ�����ף�������Ϊ�ӷ��ĵ���ɫҺ�壬���ӹ���Ϊ�����Σ��ҷ�����N��Cl����ԭ���������ﵽ8�����ӵ��ȶ��ṹ����N��Cl������ӦΪ1��3������ˮ�������γ�һ�ֳ�����Ư��������ӦΪ�����ᣬ�����ڼ�������+1�ۣ����ԼĽṹʽΪ ��
��
�ʴ�Ϊ�� ��
��
��3����������ΪNa2S��Һ�ڿ����г��ڷ��ã���������Ӧ������Na2S2����Na2S2�ĵ���ʽΪ ��Na2S��Һ�ڿ����б��ʹ��̵Ļ�ѧ����ʽΪ4Na2S+O2+2H2O�T4NaOH+2Na2S2��
��Na2S��Һ�ڿ����б��ʹ��̵Ļ�ѧ����ʽΪ4Na2S+O2+2H2O�T4NaOH+2Na2S2��
�ʴ�Ϊ�� ��4Na2S+O2+2H2O�T4NaOH+2Na2S2��
��4Na2S+O2+2H2O�T4NaOH+2Na2S2��
��XΪNԪ�أ�Ԫ��X������γɶ��ֻ��������������죬
��4��XΪNԪ�أ�Ԫ��X������γɶ��ֻ��������������죬����������ƣ�ΪһԪ���ᣬ����ķ���ʽΪHNm��HNm�ֽ�ķ���ʽΪ2HNm=H2+mN2��8.6gHNm��ը�ֽ�����H2��6.72L������£�N2������Ϊ$\frac{6.72L}{22.4L/mol}$=0.3mol����2��m=$\frac{8.6}{1+14m}$��0.3�����m=3�����Ա�ΪHN3����Ӧ����ʽΪ��2HN3=3N2+H2 ��
�ʴ�Ϊ��2HN3=3N2+H2 ��
��5����Ϊ���ӻ������ˮ��Ӧ����H2�������죬���������ܶ�Ϊ0.76g•L-1���������Է�������Ϊ0.76��22.4=17��������Ϊ��������ΪNH4H��
�ʴ�Ϊ��NH4H��
��Fe3+����Һ�зֲ�ˮ�⣬��һ��ˮ��̶������ˮ��̶����μ�С��ˮ�ⷴӦ��ƽ�ⳣ�� K1��K2��K3 �ɴ�С��˳���ǣ�K1��K2��K3��
�ʴ�Ϊ��K1��K2��K3��
���� ���⿼��Ԫ�ػ������ƶϣ��ƶ�Ԫ���ǽ���ؼ����������漰��������ѧ�������漰�����ؿ���ѧ��֪ʶǨ��Ӧ������������������Ѷ��еȣ�


 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
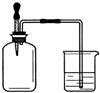 ����ͼ��ʾ������ƿ �ڳ���ij������壬���ڹ����������ι��ڵ�ˮ���뼯��ƿ���ձ��е�ˮ����뼯��ƿ������ƿ�������ǣ�������
����ͼ��ʾ������ƿ �ڳ���ij������壬���ڹ����������ι��ڵ�ˮ���뼯��ƿ���ձ��е�ˮ����뼯��ƿ������ƿ�������ǣ�������| A�� | �٢� | B�� | �ڢ� | C�� | �ڢ� | D�� | �ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã���֪��Na2S2O3��������Һ�в����ȶ����ڣ�
��������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã���֪��Na2S2O3��������Һ�в����ȶ����ڣ�| ��� | 1 | 2 | 3 | 4 |
| ��Һ�����/mL | 10.00 | 10.00 | 10.00 | 10.00 |
| ����I2����Һ�����/mL | 19.99 | 19.98 | 17.13 | 20.03 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���ữѧʽ | CH3COOH | HCN | H2CO3 |
| ����ƽ�ⳣ����25�棩 | 1.8��l0-5 | 4.9��l0-10 | K1=4.3��l0-7K2=5.6��l0-11 |
| A�� | ��NaCN ��Һ��ͨ������CO2���������ӷ�ӦΪ��2CN-+H2O+CO2�T2HCN+CO32- | |
| B�� | �����ʵ���Ũ�ȵĸ���ҺpH��ϵΪ��pH��NaCN����pH��Na2CO3����pH��CH3COONa�� | |
| C�� | a mol/LHCN��Һ��b mol/LNaOH��Һ�������Ϻ�������Һ��c��Na+����c��CN-������bһ���� ��a | |
| D�� | NaHCO3��Na2CO3�Ļ����Һ�У�һ�����ڣ�c��Na+��+c��H+��=c��OH-��+c��HCO3-��+2c��CO32-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� c��HCO3-��=0.1 mol•L-1 ����Һ�У�NH4+��AlO2-��Cl-��NO3- | |
| B�� | ����ˮ������� c��H+��=l��l0-12 mol•L-1 ����Һ�У�Cu2+��ClO-��Na+��SO42- | |
| C�� | �ڼ������۲��� H2 ����Һ�У�SO42-��NO3-��Na+��NH4+ | |
| D�� | ��ʹ��ɫʯ����ֽ��������Һ�У�SiO32-��CO32-��Na+��F- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
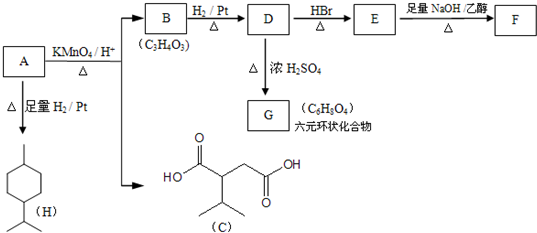
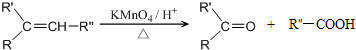
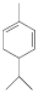 ��
��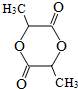 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | �¶� | KI��Һ | H2SO4��Һ | ������Һ | ʵ��Ŀ�� | ||
| C��KI�� | V | C��H2SO4�� | V | ||||
| 1 | 298K | 1mol/L | 5mL | 0.1mol/L | 5mL | 3�� | l���2��̽�����¶ȶԸ÷�Ӧ���ʵ�Ӱ�죻1���3��̽����Ӧ��Ũ�ȶԸ÷�Ӧ���ʵ�Ӱ�� |
| 2 | 308K | 1mol/L | 5mL | 0.1mol/L | 5mL | 3�� | |
| 3 | 298K | 1mol/L | 5mL | ��0.2mol/L���� | 5mL | 3�� | |
| ʵ�鷽�� | Ԥ��ʵ����������� |
| ȡ������ˮ���Թ��У����뼸�ε��� ��Һ��Ȼ����μ���1mol/L KOH��Һ���۲����� | ����ɫ����ɫ����������ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol KClO3 �μӷ�Ӧ��2mol����ת�� | |
| B�� | ClO2���������� | |
| C�� | H2C2O4��������ǿ��ClO2�������� | |
| D�� | KClO3 �ڷ�Ӧ�еõ����ӣ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ˮ���������H+Ũ��Ϊ1��10-12mol•L-1����Һ��NH4+��Na+��Cl-��HCO3- | |
| B�� | ��ʹpH��ֽ������ɫ����Һ��Na+��AlO2-��S2-��CO32- | |
| C�� | ���д���Fe3+����Һ��SCN-��I-��K+��Br- | |
| D�� | pH=1��ˮ��Һ�У�Al3+��NH4+��CH3COO-��Br- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com