
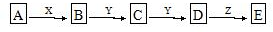
���� ��Ŀ��C��D�ı仯��D����Է���������C�Ĵ�16������һ�������������������ͨ�������ɳ����ж�D��C��һ����ԭ�ӣ�AΪ��NH4��2S�������ѹ�������ѧ��ѧ֪ʶ���磬��������ת����ϵ���У�SO2��SO3��NO��NO2��Na2SO3��Na2SO4�ȣ��ɴ˿ɳ��ƶ�YΪO2������EΪ�ᣬ��DӦΪ��ת��Ϊ���ij���ʣ��ܿ���ΪSO3��NO2�ȣ�
��X��ǿ��ʱA��B��C��D��E����ͬһ��Ԫ�أ���BΪH2S��CΪSO2��DΪSO3��EΪH2SO4��ZΪH2O����X��ǿ��ʱ����BΪNH3��CΪNO��DΪNO2��EΪHNO3��ZΪH2O���ݴ˴��⣮
��� �⣺��Ŀ��C��D�ı仯��D����Է���������C�Ĵ�16������һ�������������������ͨ�������ɳ����ж�D��C��һ����ԭ�ӣ�AΪ��NH4��2S�������ѹ�������ѧ��ѧ֪ʶ���磬��������ת����ϵ���У�SO2��SO3��NO��NO2��Na2SO3��Na2SO4�ȣ��ɴ˿ɳ��ƶ�YΪO2������EΪ�ᣬ��DӦΪ��ת��Ϊ���ij���ʣ��ܿ���ΪSO3��NO2�ȣ�
��X��ǿ��ʱA��B��C��D��E����ͬһ��Ԫ�أ���BΪH2S��CΪSO2��DΪSO3��EΪH2SO4��ZΪH2O����X��ǿ��ʱ����BΪNH3��CΪNO��DΪNO2��EΪHNO3��ZΪH2O��
��1��������C��D�ı仯��D����Է���������C�Ĵ�16������һ�������������������ͨ�������ɳ����ж�D��C��һ����ԭ�ӿ�֪AΪ��NH4��2S��YΪO2��ZΪH2O��
�ʴ�Ϊ����NH4��2S��O2��H2O��
��2����X��ǿ��ʱ����������ķ�����֪��E�� H2SO4��B����C�Ļ�ѧ����ʽΪ2H2S+3O2�T2SO2+2H2O��
�ʴ�Ϊ��H2SO4��2H2S+3O2�T2SO2+2H2O��
��3����X��ǿ��ʱ����������ķ�����֪��E�� HNO3��B����C�Ļ�ѧ����ʽΪ4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
�ʴ�Ϊ��HNO3��4NH3+5O2$\frac{\underline{����}}{��}$4NO+6H2O��
���� ���⿼��������ƶϣ���Ŀ�Ѷ��еȣ�������Ľ��һ�������²��裺˼ά����ѡ��˼ά�����ָ��ʼʱ��˼άָ��˼ά�����˼ά�Ƕȣ��ƶ����е�˼ά���Ӧ�����������ij����������������������ij���仯���̣���˼ά���̵�չ������������ȷ��˼ά���Ļ����ϣ�������Ŀ������Ϣ��������еĻ�ѧ֪ʶ�ͽ��⾭�飬���ϵ���С����״̬��Ŀ��״̬�ľ��룻˼ά���̵ļ��飺������˼ά���̵Ľ���������У����һ���Ƿ��������������


 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ú�ĸ����������仯 | |
| B�� | ú��������Һ����ҪĿ����Ϊ�˻�ýϽྻ����Դ | |
| C�� | ������Ҫ����ʯ�͵��ѽ� | |
| D�� | ��ϩ������ʯ�͵��ѻ����ѽ����Ҫ����ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͬ���칹�����࣬�����������������ҽṹ���б����ṹ��ͬ���칹�����6�֣������ǣ�
��ͬ���칹�����࣬�����������������ҽṹ���б����ṹ��ͬ���칹�����6�֣������ǣ�


 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| װ �� | �� �� |
 | ��ʵ���ʼ��δ���������� |
| ��һ������������ݣ�Һ���Ϸ���dz��ɫ | |
| ���Թܱ��ȣ���Һ���� |
| ʵ �� | �� �� | �� �� |
| ʵ��1 | ��ʪ��KI-������ֽ���ڿ����� | δ���� |
| ʵ��2 | ��ʪ��KI-������ֽ����dz��ɫ���� | ��ֽ���� |
| װ�� | ���� |
 | ��ʵ���ʼ��δ���������� |
| ��һ������������ݣ��д̼�����ζ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij�¶�ʱ�����ݻ�Ϊ2L���ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��仯��������ͼ��ʾ����ͼ�е����ݷ������÷�Ӧ�Ļ�ѧ����ʽΪ3X+Y?2Z��
ij�¶�ʱ�����ݻ�Ϊ2L���ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��仯��������ͼ��ʾ����ͼ�е����ݷ������÷�Ӧ�Ļ�ѧ����ʽΪ3X+Y?2Z���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 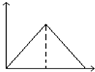 | B�� | 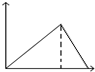 | C�� | 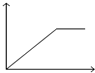 | D�� |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʳƷ���Ӽ��������к�����Ӧ����ʳƷ���������Ӽ� | |
| B�� | ������������ζ�����Ը��ƻ�ı�ʳƷ�Ŀ�ζ����������������ƻ���ᡢ������� | |
| C�� | ���������ƹ����У������������ο���ʹ���ƺõ�������Ʒ�������ĺ�ɫ�����Ӧ�ü���������������� | |
| D�� | ijЩʳƷ��װ������һ��С�������С���Ҫ�ɷ�Ϊ�����ƣ�����ʳ�á�����������������������ֹʳƷ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | MgCl2��Ħ������Ϊ95g | |
| B�� | ���³�ѹ�£�1molCO2��������44g | |
| C�� | ��״���£�1molH2O��ռ���ԼΪ22.4L | |
| D�� | 100mL 1mol/L��ϡ�����к���H+����ĿԼΪ6.02��10 22 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��2H2��g��+O2��g��=2H2O��g����H=-483.6kJ•mol-1����H2ȼ���ȣ���H��Ϊ-241.8kJ•mol-1 | |
| B�� | ��ϡ��Һ�У�H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ/mol��������0.6molH2SO4��ϡ�����뺬1molNaOH����Һ��ϣ��ų�����������57.3kJ | |
| C�� | ��֪C��ʯī��s���TC�����ʯ��s����H��0������ʯ��ʯī�ȶ� | |
| D�� | ��BaSO4��s��+4C��s��=4CO��g��+BaS��s����H1=+571.2kJ•mol-1��BaSO4��s��+2C��s��=2CO2��g��+BaS��s����H2=+226.2kJ•mol-1�ڿɵ÷�ӦC��s��+CO2��g��=2CO��g���ġ�H=+172.5kJ•mol-1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com