
 ijʵ��С������50mL NaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ���CO2�����������NaHCO3�����������ʵ�鲽�裺
ijʵ��С������50mL NaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ���CO2�����������NaHCO3�����������ʵ�鲽�裺


 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �ζ����� ʵ������ |
1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.98 | 15.20 | 15.12 | 15.95 |
| V��NaOH��/mL�����ģ� | 14.98 | 15.00 | 15.02 | 15.95 |
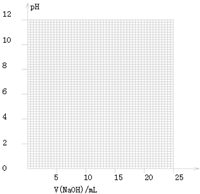
| V��NaOH��/mL | 0.00 | 10.00 | 18.00 | 19.80 | 19.98 | 20.00 | 20.02 | 20.20 | 22.00 |
| ��ҺpH | 2.88 | 4.70 | 5.70 | 6.74 | 7.74 | 8.72 | 9.70 | 10.70 | 11.70 |
| ָʾ�� | ��ɫ�ķ�Χ��pH�� |
| ���� | 3.1��4.4 |
| ʯ�� | 5.0��8.0 |
| ��̪ | 8.2��10.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L����������Һ��ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�����ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺
ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L����������Һ��ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�����ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶ȣ�t2���� | �²t2-t1���� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ζ����� ʵ������ |
1 | 2 | 3 | 4 |
| V����Ʒ��/mL | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH��/mL���������� | 0.00 | 0.200 | 0.10 | 0.00 |
| V��NaOH��/mL���ն����� | 14.98 | 15.20 | 15.12 | 15.95 |
| V��NaOH��/mL�����ģ� | 14.98 | 15.00 | 15.02 | 15.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijʵ��С������50 mL NaOH��Һ����CO2���壬�Ʊ�Na2CO3��Һ��Ϊ�˷�ֹͨ���CO2�����������NaHCO3�����������ʵ�鲽�裺
����25 mLNaOH��Һ����CO2���壬��CO2���岻���ܽ⣻
��С�������Һ1min~2min��
���ڵõ�����Һ�м�����һ�루25 mL��NaOH��Һ��ʹ���ֻ�ϡ�
�˷������Ƶýϴ�����Na2CO3����һ����ʵ��װ����ͼ��ʾ��

��1������״����ʯ�����Թ��е���ȷ������ _____________��
��2��д���١��������Ļ�ѧ��Ӧ����ʽ _____________��
��3��װ��B��ʢ�ŵ��Լ���_____________��������_____________��
��4��������Ϊ��ʵ�鲽��ڡ��۵�˳��Ե������Ȼ������У�������������Ϊ����
_____________����ԡ����ԡ��������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com