
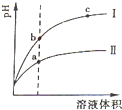
| c(H+) |
| c(CH3COOH) |
| 10-3 |
| 0.1 |
| 10-14 |
| 10-3 |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
| ��þ�� | ��ɰ | ���� | ƫ������ |
| Mg2B2O5?H2O | Na2B4O7?10H2O | H3BO3 | NaBO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ƽ�ⳣ����K��ԽС����ʾ������ʵ�������Խ�� |
| B������ƽ�ⳣ����K�����¶��� |
| C����ͬŨ�ȵ�ͬһ������ʣ������ƽ�ⳣ����K����ͬ |
| D����Ԫ�����������ƽ�ⳣ�����ϵΪ��K1��K2��K3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ڢ� | C���ۢ� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������£���pH=3�Ĵ�����Һϡ�͵�ԭ�����10������Һ��pHС��4 |
| B��Ϊȷ��ij��H2A��ǿ�ỹ�����ᣬ�ɲ�NaHA��Һ��pH����pH��7����H2A�������pH��7����H2A��ǿ�� |
| C����0.200mol?L-1NaOH����Һ�ζ�HCl��CH3COOH�Ļ��Һ�����Һ���������Ũ�Ⱦ�ԼΪ0.1mol?L-1����������ʱ����Һ�е���δ����ȫ�к� |
| D����ͬ�¶��£��������Ȼ�������ֱ������ͬ����Ģ�����ˮ����0.1mol?L-1���ᡢ��0.1mol?L-1�Ȼ�þ��Һ����0.1mol?L-1��������Һ�У�Ag+Ũ�ȣ��ܣ��٣��ڣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ʵ����ǿ��������ͬ�¶��µĵ��볣��ֵ�Ĵ�С���ж� |
| B��ͬһ������ʣ�Ũ��Խ����̶�Խ�� |
| C�������¶ȣ�������ʵĵ���̶ȼ�С |
| D��ͨ���������Խ����������Һ�ĵ���������Խ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����������� | B��������� | C��H+ | D��OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ܢۢڢ� | B���٢ڢۢ� | C���ۢڢܢ� | D���ۢܢڢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com