
ЁОЬтФПЁП
ЃЈ1ЃЉгУЭаХЬЬьЦНГЦШЁNaOHЙЬЬх____________gЁЃГЦСПNaOHЙЬЬхашзЂвтЃКвђNaOHОпгаИЏЪДадЃЌдкГЦСПЪБашгУЩеБЪЂзАNaOHЙЬЬхЃЛГЦСПБиаыбИЫйЃЌЦфдвђЪЧ_________________________ЁЃ
ЃЈ2ЃЉ НгЯТРДЭъГЩДЫЪЕбщФуНЋгУЕНЕФвЧЦїгаВЃСЇАєЁЂЩеБЁЂ_________________ЁЃ
ЖјФГЭЌбЇЬсГіжЛашдйбЁдёДјПЬЖШЕФ500 mLЩеБКЭВЃСЇАєЃЌМДПЩХфжЦ500 mL 0.10 mol/LЕФNaOHШмвКЃЛФуЖдДЫЗНАИЕФЦРМлЪЧ_______________________ЁЃ
ЃЈ3ЃЉ ШєФуДгЫљХфЕФШмвКжаШЁГі50 mLгквЛЪдМСЦПжаЃЌЧыИјЫќЬљЩЯБъЧЉЃЌБъЧЉЕФФкШнЪЧ_________ЃЛ
ШєдйДгжаШЁГі10 mLШмвКМгЫЎЯЁЪЭжС20 mLЃЌдђДЫЪБШмвКЕФЮяжЪЕФСПХЈЖШЮЊ____________ЁЃ
ЃЈ4ЃЉ ЯТСаВйзїЖдЫљХфШмвКХЈЖШУЛгагАЯьЕФЪЧ_______ЁЃ
AЃЎГЦСПЪБвбЙлВьЕНNaOHБэУцГБЪЊ |
BЃЎНЋЩеБжаШмНтКѓЕФШмвКСЂМДзЂШыШнСПЦПЃЌШЛКѓдйЬэМгеєСѓЫЎжСПЬЖШЯп |
CЃЎвЁдШЖЈШнКѓЃЌгжгУНКЭЗЕЮЙмЯђШнСПЦПжаЕЮМгеєСѓЫЎжСПЬЖШЯп |
DЃЎХфжЦШмвКЧАгУеєСѓЫЎШѓЯДШнСПЦПЃЌЕЋЮДКцИЩ |
ЁОД№АИЁПЃЈ1ЃЉ 2.0ЃЛ ЗРжЙГЦСПЪБМфЙ§ГЄЃЌNaOHЮќЫЎЃЛ
ЃЈ2ЃЉ 500 mLШнСПЦПЁЂНКЭЗЕЮЙмЃЛгЩгк500 mLЩеБЫљСПЬхЛ§ВЂВЛОЋШЗЃЌИУЭЌбЇЫљХфШмвКЕФХЈЖШжЛЪЧдМЮЊ0.1 mol/LЃЛ
ЃЈ3ЃЉ 0.10 mol/L NaOH(aq)ЃЛ0.05 mol/LЃЛ
ЃЈ4ЃЉ DЃЛ
ЁОНтЮіЁПЪдЬтЗжЮіЃКЃЈ1ЃЉашЧтбѕЛЏФЦЕФжЪСПЮЊЃКm(NaOH)=0.5LЁС0.1molL-1ЁС40g/mol=2.0gЃЛЧтбѕЛЏФЦгаИЏЪДадвзГБНтгІЗХдкаЁЩеБФкбИЫйГЦСПЃЌЗРжЙГЦСПЪБМфЙ§ГЄЃЌNaOHЮќЫЎЃЌЙЪД№АИЮЊЃК2.0ЃЛЗРжЙГЦСПЪБМфЙ§ГЄЃЌNaOHЮќЫЎЃЛ
ЃЈ2ЃЉХфжЦ500mLЕФЧтбѕЛЏФЦШмвКЃЌашвЊбЁгУ500mLШнСПЦПЃЌЖЈШнЪББиаыЪЙгУНКЭЗЕЮЙмЖЈШнЃЌЫљвдЛЙШБЩй500mLШнСПЦПЁЂНКЭЗЕЮЙмЃЛбЁдёДјПЬЖШЕФ500mLЩеБКЭВЃСЇАєХфжЦNaOHШмвКЃЌгЩгк500mLЩеБЫљСПЬхЛ§ВЂВЛОЋШЗЃЌИУЭЌбЇЫљХфШмвКЕФХЈЖШжЛЪЧдМЮЊ0.1 mol/LЃЌЙЪД№АИЮЊЃК500mLШнСПЦПЁЂНКЭЗЕЮЙмЃЛгЩгк500mLЩеБЫљСПЬхЛ§ВЂВЛОЋШЗЃЌИУЭЌбЇЫљХфШмвКЕФХЈЖШжЛЪЧдМЮЊ0.1 mol/LЃЛ
ЃЈ3ЃЉДгЫљХфЕФШмвКжаШЁГі50mLгквЛЪдМСЦПжаЃЌБъЧЉЩЯгІИУзЂУїШмвКЕФУћГЦМАХЈЖШЃЌИУЧтбѕЛЏФЦШмвКЕФБъЧЉЕФФкШнЮЊЃК0.10 mol/L NaOH(aq)ЃЛШєдйДгжаШЁГі10mLШмвКМгЫЎЯЁЪЭжС20mLЃЌИљОнn=cVПЩжЊЃЌХЈЖШгыШмвКЕФЬхЛ§ГЩЗДБШЃЌЬхЛ§РЉДѓЮЊдЯШЕФ2БЖЃЌдђДЫЪБШмвКЕФЮяжЪЕФСПХЈЖШЮЊ0.01mol/LЁС![]() =0.05mol/LЃЌЙЪД№АИЮЊЃК0.10 mol/L NaOH(aq)ЃЛ0.05 mol/LЃЛ
=0.05mol/LЃЌЙЪД№АИЮЊЃК0.10 mol/L NaOH(aq)ЃЛ0.05 mol/LЃЛ
ЃЈ4ЃЉAЃЎГЦСПЪБвбЙлВьЕНNaOHБэУцГБЪЊЃЌЕМжТХфжЦЕФШмвКжаЧтбѕЛЏФЦЕФЮяжЪЕФСПЦЋаЁЃЌХфжЦЕФШмвКХЈЖШЦЋЕЭЃЌЙЪAДэЮѓЃЛBЃЎНЋЩеБжаШмНтКѓЕФШмвКСЂМДзЂШыШнСПЦПЃЌШЛКѓдйЬэМгеєСѓЫЎжСПЬЖШЯпЃЌУЛгаРфШДЃЌШШЕФШмвКЬхЛ§ЦЋДѓЃЌРфШДКѓШмвКЬхЛ§БфаЁЃЌЕМжТХфжЦЕФШмвКЬхЛ§ЦЋаЁЃЌХфжЦЕФШмвКХЈЖШЦЋИпЃЌЙЪBДэЮѓЃЛCЃЎвЁдШЖЈШнКѓЃЌгжгУНКЭЗЕЮЙмЯђШнСПЦПжаЕЮМгеєСѓЫЎжСПЬЖШЯпЃЌДѓЦзгМгШыЕФеєСѓЫЎЬхЛ§ЦЋДѓЃЌХфжЦЕФШмвКЬхЛ§ЦЋДѓЃЌШмвКХЈЖШЦЋЕЭЃЌЙЪCДэЮѓЃЛDЃЎХфжЦШмвКЧАгУеєСѓЫЎШѓЯДШнСПЦПЃЌЕЋЮДКцИЩЃЌЖдШмжЪЕФЮяжЪЕФСПМАШмвКЕФЬхЛ§ЖМУЛгагАЯьЃЌЫљвдВЛгАЯьХфжЦНсЙћЃЌЙЪDе§ШЗЃЛЙЪД№АИЮЊЃКDЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПДМЪЧживЊЕФгаЛњЛЏЙЄдСЯЁЃвЛЖЈЬѕМўЯТЃЌМзДМПЩЭЌЪБЗЂЩњЯТУцСНИіЗДгІЃК
i .2CH3OHЃЈgЃЉ![]() CH3OCH3ЃЈgЃЉЃЋH2OЃЈgЃЉ
CH3OCH3ЃЈgЃЉЃЋH2OЃЈgЃЉ
ii.2CH3OHЃЈgЃЉ![]() C2H4ЃЈgЃЉЃЋ2H2OЃЈgЃЉ
C2H4ЃЈgЃЉЃЋ2H2OЃЈgЃЉ
IЃЎЩЯЪіЗДгІЙ§ГЬжаФмСПБфЛЏШчЭМЫљЪОЃК

ЃЈ1ЃЉдкФГУмБеШнЦїжаЃЌГфШывЛЖЈСПCH3OHЃЈgЃЉЗЂЩњЩЯЪіСНИіЗДгІЃЌЗДгІ________ЃЈЬюЁАiЁБЛђЁАiiЁБЃЉЕФЫйТЪНЯДѓЃЌЦфдвђЮЊ___________ЁЃШєдкШнЦїжаМгШыДпЛЏМСЃЌЪЙiiЕФЗДгІЫйТЪдіДѓЃЌдђE1КЭE2-E1ЕФБфЛЏЪЧЃКE1__________ЃЛE2ЃE1___________ЃЈЬюЁАдіДѓЁБЁЂЁАМѕаЁЁБЛђЁАВЛБфЁБЃЉЁЃ
ЃЈ2ЃЉвбжЊЃКCH3CH2OHЃЈgЃЉ=CH3OCH3ЃЈgЃЉ ІЄH =ЃЋ50.7 kJЁЄmolЃ1ЁЃдђввЯЉЦјЯржБНгЫЎКЯЗДгІC2H4ЃЈgЃЉЃЋH2OЃЈgЃЉ=C2H5OHЃЈgЃЉЕФІЄH= ЁЃ
ЃЈ3ЃЉШєдкШнЛ§ЮЊ2 LЕФКуШнУмБеШнЦїжаЗЂЩњi ЁЂiiЗДгІЃЌЕБЦ№ЪМЭЖСЯЮЊ2 mol CH3OHЃЈgЃЉ ЕФЯћКФСПЮЊ80%ЪБШнЦїжаЦјЬхЕФЦНОљЯрЖдЗжзгжЪСПЮЊ ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭМЫљЪОЕФЮяжЪЗДгІЙиЯЕжаЃЌВПЗжВњЮяБЛТдШЅЁЃвбжЊ2 molАзЩЋЙЬЬхЗлФЉXЪмШШЗжНтЃЌЛжИДЕНЪвЮТЩњГЩАзЩЋЙЬЬхAЁЂЮоЩЋвКЬхBЁЂЮоЩЋЦјЬхCИї1 molЁЃAЁЂXЁЂEЁЂGЁЂHЕФбцЩЋЗДгІОљЮЊЛЦЩЋЃЌЧвHЮЊЕЛЦЩЋЙЬЬхЁЃ

ЛиД№ЯТСаЮЪЬтЃК
(1)аДГіЯТСаЮяжЪЕФЛЏбЇЪНЃКX________ЃЛ H________ЁЃ
(2)аДГіE+AlЁњGЗДгІЕФЛЏбЇЗНГЬЪН_________ЃЛ
(3)аДГіGШмвКжаЭЈШыЙ§СПCжЦШЁDЕФЗДгІРызгЗНГЬЪНЃК____________________ЃЛ
(4)аДГіXШмвКжаЕЮМгЙ§СПГЮЧхЪЏЛвЫЎЗДгІЕФРызгЗНГЬЪНЃК__________________ЃЛ
(5)0.1 mol HгызуСПЕФCЗДгІЃЌзЊвЦЕФЕчзгЪ§ЮЊ________ИіЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПAЁЋGЦпжжЮяжЪЕФзЊЛЏЙиЯЕШчЭМЫљЪО(ВПЗжЗДгІЮяЁЂВњЮяКЭЗДгІЬѕМўЮДБъГі)ЁЃЦфжаЃЌAЁЂBЮЊжабЇЛЏбЇГЃМћЕФН№ЪєЕЅжЪЃЌCЪЧЕЛЦЩЋЙЬЬхЃЌDМШФмгыЧПЫсШмвКЗДгІЃЌгжФмгыЧПМюШмвКЗДгІЃЌFШмвКжаМгШыAgNO3ШмвКВњЩњВЛШмгкЯЁЯѕЫсЕФАзЩЋГСЕэЃЌEКЭGбцЩЋЗДгІОљГЪЛЦЩЋЁЃЂйЁЂЂмЁЂЂнОљЮЊШМЩеЗДгІЁЃ
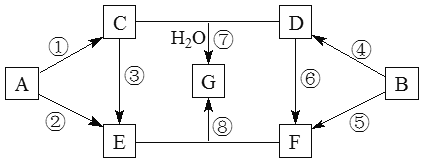
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉFЕФЛЏбЇЪН ЃЌGЕФЛЏбЇЪН ЁЃ
ЃЈ2ЃЉЗДгІЂйЕФЛЏбЇЗНГЬЪНЪЧ ЁЃ
ЃЈ3ЃЉаДГіЯТСаЗДгІЕФРызгЗНГЬЪНЃК
Ђл ЃЌЂо ЁЃ
ЃЈ4ЃЉНЋEЕФШмвКж№ЕЮМгШыЕНFЕФШмвКжажСЙ§СПЃЌЦфЯжЯѓЪЧ ЃЌ ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЮЊСЫбаОПЬМЫсИЦгыбЮЫсЗДгІЕФЗДгІЫйТЪЃЌФГЭЌбЇЭЈЙ§ШчЭМЪЕбщзАжУВтЖЈЗДгІжаЩњГЩЕФCO2ЦјЬхЬхЛ§ЃЌВЂЛцжЦГіШчЭМЫљЪОЕФЧњЯпЁЃЧыЗжЮіЬжТлвдЯТЮЪЬтЁЃ


ЃЈ1ЃЉЛЏбЇЗДгІЫйТЪзюПьЕФЪБМфЖЮЪЧ ЃЌгАЯьДЫЪБМфЖЮЗДгІЫйТЪЕФжївЊвђЫиЪЧ ЃЛ
AЃЎOЁЋt1 BЃЎt1ЁЋt2 CЃЎt2ЁЋt3 DЃЎt3ЁЋt4
ЃЈ2ЃЉЮЊСЫМѕЛКЩЯЪіЗДгІЫйТЪЃЌгћЯђбЮЫсжаМгШыЯТСаЮяжЪЃЌФуШЯЮЊПЩааЕФга ЃЛ
AЃЎеєСѓЫЎ BЃЎNaClЙЬЬх CЃЎNaClШмвК DЃЎЭЈШыHCl
ЃЈ3ЃЉШєбЮЫсЕФЬхЛ§ЪЧ20 mLЃЌЭМжаCO2ЕФЬхЛ§ЪЧБъзМзДПіЯТЕФЬхЛ§ЃЌдђt1~t2ЪБМфЖЮЦНОљЗДгІЫйТЪv(HCl)= molЁЄ(LЁЄmin)-1ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЩшNAБэЪОАЂЗќйЄЕТТоГЃЪ§ЕФжЕЃЌЯТСаЫЕЗЈжае§ШЗЕФЪЧ
A. 200g63%ЕФХЈЯѕЫсжаКЌбѕдзгИіЪ§ЮЊ6NA
B. 50mL12molЁЄL-1бЮЫсКЭзуСПMnO2ЙВШШЃЌзЊвЦЕФЕчзгЪ§ФПЮЊ0.3NA
C. ЧтбѕШМСЯЕчГие§МЋЯћКФ22.4L(БъПіЯТ)ЦјЬхЪБЃЌЕчТЗжаЭЈЙ§ЕФЕчзгЪ§ФПЮЊ2NA
D. ШєCH3COONaШмвКжаCH3COO-ЕФЪ§ФПЮЊ6NAЃЌдђNa+Ъ§ФПДѓгк6NA
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЗДгІ4NH3(g)ЃЋ5O2(g)![]() 4NO(g)ЃЋ6H2O(g)дк5LУмБеШнЦїжаНјааЃЌАыЗжжгКѓNOЕФЮяжЪЕФСПдіМгСЫ0.3 molЃЌдђДЫЗДгІЕФЦНОљЫйТЪvЮЊ( )
4NO(g)ЃЋ6H2O(g)дк5LУмБеШнЦїжаНјааЃЌАыЗжжгКѓNOЕФЮяжЪЕФСПдіМгСЫ0.3 molЃЌдђДЫЗДгІЕФЦНОљЫйТЪvЮЊ( )
AЃЎv(O2)ЃН0.01 molЁЄLЃ1ЁЄsЃ1 BЃЎv(NO)ЃН0.08 molЁЄLЃ1ЁЄsЃ1
CЃЎv(H2O)ЃН0.003 molЁЄLЃ1ЁЄsЃ1 DЃЎv(NH3)ЃН0.001 molЁЄLЃ1ЁЄsЃ1
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПМзЁЂввЪЧжмЦкБэжаЭЌвЛжмЦкЕФСНжждЊЫиЃЌМздкЂђAзхЃЌввдкЂєAзхЃЌФЧУДМзЁЂввСНдЊЫиЕФдзгађЪ§жЎВюВЛПЩФмЪЧЃЈ ЃЉ
A.2
B.12
C.26
D.11
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдкШчЭМгУЪЏФЋзїЕчМЋЕФЕчНтГижаЃЌЗХШы500mLКЌвЛжжШмжЪЕФФГРЖЩЋСђЫсбЮШмвКНјааЕчНтЃЌЙлВьЕНAЕчМЋБэУцгаКьЩЋЕФЙЬЬЌЮяжЪЩњГЩЃЌBЕчМЋгаЮоЩЋЦјЬхЩњГЩЁЃЧыЛиД№ЯТСаЮЪЬтЃК

ЃЈ1ЃЉBМЋАхЕФУћГЦЪЧ_____________ЃЌЕчНтКѓШмвКЯд_________ЃЈЬюЁАЫсадЁБЛђЁАМюадЁБЃЉаДГіЕчНтЪБЗДгІЕФзмРызгЗДгІЗНГЬЪНЮЊ ________________________
ЃЈ2ЃЉШєЕБШмвКжаЕФдгаШмжЪЭъШЋЕчНтКѓЃЌЭЃжЙЕчНтЃЌШЁГіAЕчМЋЃЌЯДЕгЁЂИЩдяЁЂГЦСПЁЂЕчМЋдіжи1.6gЁЃвЊЪЙЕчНтКѓШмвКЛжИДЕНЕчНтЧАЕФзДЬЌЃЌдђашМгШы___________ЃЌЦфжЪСПЮЊ__________gЁЃЃЈМйЩшЕчНтЧАКѓШмвКЕФЬхЛ§ВЛБфЃЉ
ЃЈ3ЃЉШєвЊгУЕчЖЦЗНЗЈдкЬњБэУцЖЦвЛВуН№ЪєвјЃЌгІИУбЁдёЕФЗНАИЪЧ
ЗНАИ | A | B | ЕчНтжЪШмвК |
A | вј | ЪЏФЋ | AgNO3 |
B | вј | Ьњ | AgNO3 |
C | Ьњ | вј | Fe(NO3)3 |
D | Ьњ | вј | AgNO3 |
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com