
�����ӵ�����ԼΪ6.02��1023 mol��1��������������ȷ����
A.���³�ѹ�£�18.0g ��ˮ��D2O�������ĵ�����ԼΪ10��6.02��1023
B.�����£�42.0g��ϩ�ͱ�ϩ�Ļ�������к��е�̼ԭ����Լ Ϊ3��6.02��1023
Ϊ3��6.02��1023
C.��״���£�22.4L �ױ������ķ�����ԼΪ6.02��1023
D.��״���£�a L ����������������еķ�����ԼΪ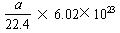
���𰸡�BD
�������������ۺϿ������ʵ���������������������ӵ�����֮ ��Ĺ�ϵ��
��Ĺ�ϵ��
1 mol D2O����10 mol ���ӣ���18.0 g D2O����������Ϊ��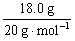 ��10��6.02��1023 mol��1��10��6.02��1023��A����ȷ����ϩ����ϩ��ʵ��ʽ��ΪCH2����42 g������������е�̼ԭ����Ϊ��
��10��6.02��1023 mol��1��10��6.02��1023��A����ȷ����ϩ����ϩ��ʵ��ʽ��ΪCH2����42 g������������е�̼ԭ����Ϊ��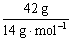 ��6.02��1023 mol��1=3��6.02��1023��B��ȷ���ױ��ڱ�״����ΪҺ�壬22.4 L�ױ������ʵ���Զ����1 mol����C����ȷ����״���£�a L CH4��C2H6�����������ʵ���Ϊ
��6.02��1023 mol��1=3��6.02��1023��B��ȷ���ױ��ڱ�״����ΪҺ�壬22.4 L�ױ������ʵ���Զ����1 mol����C����ȷ����״���£�a L CH4��C2H6�����������ʵ���Ϊ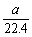 mol������������Ϊ
mol������������Ϊ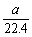 ��6.02��1023��D��ȷ���ʱ����ΪB��D��
��6.02��1023��D��ȷ���ʱ����ΪB��D��


 ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д� С�����ϵ�д�
С�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڱ�״���£�CO��CO2�Ļ�����干67.2L������Ϊ116g����
��1��������������ʵ���֮��Ϊ����mol��
��2������CO2���� mol��
��3��CO���������Ϊ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA��ʾ�����ӵ�������ֵ������������ȷ����( )
A.25 ��ʱ��pH��13��1.0 L Ba(OH)2��Һ�к��е�OH-��ĿΪ0.2NA
B.��״���£�2.24 L Cl2�����ϡNaOH��Һ��Ӧ��ת�Ƶĵ�������Ϊ0.2NA
C.�����£�21.0 g��ϩ�Ͷ�ϩ�Ļ�������к��е�̼ԭ����ĿΪ1.5NA
D.��״���£�22.4 L�״��к��е���ԭ����Ϊ1.0NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͬ״���µ�12C18O��14N2�������壬����˵����ȷ����
 A.��������ȣ������������
A.��������ȣ������������
B.��ԭ������ȣ������������
C.����������ȣ���������
D.�������ȣ����ܶ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�Ȼ�þ��Һ���ܶ�Ϊ1.18 g��cm-3������þ���ӵ���������Ϊ5.1%��300 mL����Һ��Cl�����ӵ����ʵ���Լ����
A 0.37 mol B 0.63 mol
C 0.74 mol D 1.5 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����뻯ѧ�йص�������ȷ����
A.ͭпԭ�����ͭΪ����
B.0.1 mol N2��0. 3 mol H2��һ�������·�Ӧ�ﵽƽ��ʱ������0.2 mol��
3 mol H2��һ�������·�Ӧ�ﵽƽ��ʱ������0.2 mol��
C.1 mol��L-1 AlCl3��Һ�У����������ʵ���Ũ��Ϊ1 mol��L-1
D.18 g H2O�к�1 mol��ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������и������ʵ���������ȷ����
�� H2N��CH2��COOH��H3C��CH2��NO2 �� CH3COOH��HCOOCH3
�� CH3CH2CHO �� CH3COCH3 �� CH3CH2CH2OH��CH3CH2OCH3
��2��������Ͷ��� ��CH3CH=CHCH2CH3��CH3CH2CH2CH=CH2
��CH3(CH2)4CH3 �� CH3CH(CH3)CH2CH3
A���٢ڢۢܢݢޢ߶���ͬ���칹�� B�����ǹ������칹����
C������̼���칹���� D���٢ڢۢ��ǹ������칹����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��д�����з�Ӧ�Ļ�ѧ����ʽ:
(1)ʵ �� �� �� ȡ �� Ȳ �� �� ѧ �� �� ʽ ������������������������������������������
(2)�Ҵ��Ĵ�����:
(3)д���ɱ���ϩ��  ���ڴ������������ɾ۱���ϩ�ķ�
���ڴ������������ɾ۱���ϩ�ķ�
Ӧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�鴦�������е��� �� ��
 |
�٣��� ��NaOH�Ĵ���Һ�����Ʊ�CH3��CH�TCH2
�ڣ������ͼ�ȩ�Ļ�����м�������������Һ���кͼ���������Ƶ�������ͭ���ȡ��������������Ƿ��м�ȩ
�ۣ����ϩȩ��CH2=CH��CHO���е���KMnO4(H+)��Һ���۲���ɫ��ȥ����֤���ṹ�д���̼̼˫��
�ܣ�Ϊ��֤ijRX�ǵ���飬��RX���ռ�ˮ��Һ��ϼ��Ⱥ���Һ��ȴ���ٵ��뼸����������Һ���۲�����
A��ֻ�Т٢� B��ֻ�Т٢ڢ�
C��ֻ�Тڢۢ� D��������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com