
���� һ��ɫ�����ĩ������̼���ơ��������ơ��������е�һ�ֻ���֣�����ˮ���ȼ����ᣬ��μ��Ȼ�����Һ�����ɰ�ɫ������֤�������ƵĴ��ڣ�������Ʒ������������У�������ų�������Ϊ������̼�������������Ʒ������������ȥ�������������ʯ��ˮ���������̼���Դ������
��� �⣺��1��ȡ������Ʒ�ܽ���ˮ�����ձ����ܽ⣬�����Ͻ��裬�ܽ����õIJ����������ձ������������ʴ�Ϊ���ձ�����������
��2���������Ʒ�Ƿ��������ƣ�����Ϊȡ������1������Һ�����������ᣬ��ַ�Ӧ�������������ټ�������BaCl2��Һ�����г�������˵����Ʒ����Na2SO4������������˵��˵����Ʒ��û��Na2SO4��
�ʴ�Ϊ�����������ᣬ��ַ�Ӧ�������������ټ�������BaCl2��Һ�����г�������˵����Ʒ����Na2SO4������������˵��˵����Ʒ��û��Na2SO4��
��3��������Ʒ������������У�������ų�������Ϊ������̼�������������Ʒ������������ȥ�������������ʯ��ˮ���������̼�������������ͨ��ʢ��Ʒ����Һ������KMnO4��Һ��Ʒ����Һ������ʯ��ˮ��ϴ��ƿ��Ʒ����Һ��ɫ��˵������������ԭ��������������ƣ���֮���������ʯ��ˮ����ǣ�˵����̼���ƣ���֮������
�ʴ�Ϊ��Ʒ����Һ������KMnO4��Һ��Ʒ����Һ������ʯ��ˮ��Ʒ����Һ��ɫ��˵������������ԭ��������������ƣ���֮���������ʯ��ˮ����ǣ�˵����̼���ƣ���֮������
���� ���⿼��������ɵ��ƶϣ�Ϊ��Ƶ���㣬���ճ������ӵļ��鷽��������ʱ��������۵Ĺ�ϵΪ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | A��CH2=CH2 B��CH3CHO C��CH3CH2OH | |
| B�� | A��CH3CHO B��CH2=CH2C��CH3CH2OH | |
| C�� | A��CH��CH B��CH3CH2OH C��CH3CHO | |
| D�� | A��CH3CH2OH B��CH3-CH3C��CH��CH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ƶ��۵�ϵ� | B�� | �Ƶ��ܶȽ�С | ||
| C�� | �Ƶ�ʧ����������ǿ | D�� | �Ƶĵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ�� | B�� | ��ˮ�� | C�� | ǿ������ | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����£�0.1 mol/L Na2S��Һ�д��ڣ�c��OH-��=c��H+��+c��HS-��+c��H2S�� | |
| B�� | �����£�pHΪ1��0.1 mol/L HA��Һ��0.1 mol/L NaOH��Һǡ����ȫ��Ӧʱ����Һ��һ�����ڣ�c ��Na+��=c��A-����c��OH-��=c��H+�� | |
| C�� | �����£�pH=7��CH3COONa��CH3COOH�����Һ��c��Na+��=0.1 mol/L��c��Na+��=c��CH3COOH����c��CH3COO-����c��H+��=c��OH-�� | |
| D�� | �����£���0.1 mol/L CH3COOH��Һ��ˮϡ�ͣ�����Һ��pH��3.0����4.0ʱ����Һ��Kac��H+��ֵ��С��ԭ����$\frac{1}{10}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��״���£�11.2L��O2��NO�Ļ���ﺬ�еķ�����ԼΪ0.5��6.02��1023 | |
| B�� | 1mol���ǻ���1 mol������������������������Ϊ9 NA | |
| C�� | ���³�ѹ��42g ��ϩ�Ͷ�ϩ��������У�̼�������ĿΪ6NA | |
| D�� | 6.4g SO2��3.2g������Ӧ����SO3��ת�Ƶ�����Ϊ0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
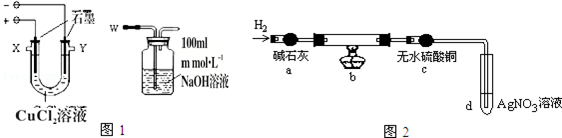
| �������Ƽ���ѧʽ | �Ȼ���ͭCuCl | ��ʽ�Ȼ�ͭCu2��OH��3Cl |
| ���� | ��ɫ���塢����ˮ | ��ɫ���塢����ˮ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com