
 __________________________________________________
__________________________________________________ ______________________��
______________________�� 1)����Һ����ȡ20.00 mL FeSO4��Һ������ƿ�У���0.10 mol��L��1������KMnO4��Һ�����յ㣬��ȥKMnO4��Һ20.00 mL������������MnԪ��ȫ���ʣ�2�ۣ��ζ���Ӧ�����ӷ���ʽΪ______ __���ݴ˿ɲ��FeSO4��Һ�����ʵ���Ũ��Ϊ______ __mol��L��1��
1)����Һ����ȡ20.00 mL FeSO4��Һ������ƿ�У���0.10 mol��L��1������KMnO4��Һ�����յ㣬��ȥKMnO4��Һ20.00 mL������������MnԪ��ȫ���ʣ�2�ۣ��ζ���Ӧ�����ӷ���ʽΪ______ __���ݴ˿ɲ��FeSO4��Һ�����ʵ���Ũ��Ϊ______ __mol��L��1��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 Bƫ�͡�C��Ӱ�죩
Bƫ�͡�C��Ӱ�죩�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢۢܢݢ� | B���ڢݢ٢ۢܢ� | C���٢ۢݢڢ� | D���ڢ٢ۢޢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
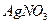 ��
��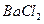 ��
��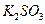 ��
��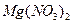 ������Һ������������֮������Ӧ�����Լ����� �� ��
������Һ������������֮������Ӧ�����Լ����� �� ��| A�����ᡢ���� | B�� ���ᡢ����������Һ ���ᡢ����������Һ |
| C����ˮ������ | D����ˮ������������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A����ȴ | B������ | C��ϴ�� | D������ E���ܽ� F��ҡ�ȡ� G����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ��������FeCl3��MgCl2��Һ�м�������Mg(OH)2��ĩ������һ��ʱ������ | ��ȥMgCl2��Һ��������FeCl3 |
| B | ��ij��Һ�м���BaCl2��Һ���ɰ�ɫ������������ϡ�����������ʧ | ֤����Һ�к�SO42- |
| C | ��ij��Һ�м���ϡ���ᣬ�ų���ɫ��ζ�����壬������ͨ�����ʯ��ˮ��ʯ��ˮ����� | ֤������Һ�д���CO32- |
| D | ��0.1mol/LFe SO4��Һ�еμ���������KMnO4��Һ��KMnO4��Һ��ɫ | ֤��Fe2+���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
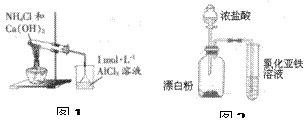
| A����ȥKCl��Һ�л��е�BaCl2�������Լ���˳�� Na2CO3��Һ��HCl��Һ |
| B��ͼ1�ձ����ȳ��ְ�ɫ�������ܽ� |
| C������ͼ2װ����֤�����ԣ�ClO-��Cl2��Fe3+ |
| D����NaHSO3��Һ�е���ʯ����Һ����֤��ҺNaHSO3�д���ˮ��ƽ�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com