
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
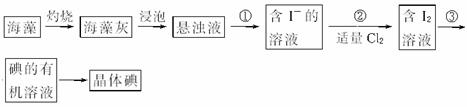
 ����___________ (�����)��Һ����Ӧ�����ӷ���ʽΪ______________________��
����___________ (�����)��Һ����Ӧ�����ӷ���ʽΪ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | ʵ���������ʵ | ʵ��Ŀ�Ļ���� |
| A | ����ɫ��Һ ���ɫ���� ���ɫ���� | ˵��ԭ��Һ��һ������FeCl3 |
| B | Br2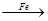 FeBr3 FeBr3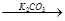 KBr KBr | �Ʊ�����KBr��Һ |
| C | ��ɫ��Һ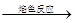 ��ɫ�ʻ�ɫ ��ɫ�ʻ�ɫ | ˵��ԭ��Һ��һ��������Ԫ�� |
| D | H3PO3+2NaOH(����)=Na2HPO3+2H2O | H3PO3������Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
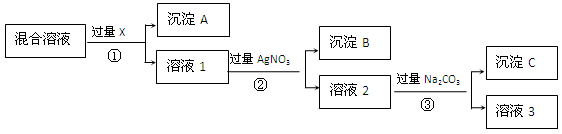
�鿴�𰸺ͽ���>>
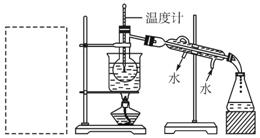
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ���¿̶ȡ�
���¿̶ȡ� Щʵ��
Щʵ�� ��������� ��
��������� ��| A��ת�ƴ���Һ������ƿʱ��δϴ���ձ� |
| B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ���� |
| C���ζ�ʱ����Ӧ����ҡ��̫���ң�����������Һ���� |
| D���ζ����յ�ʱ���ζ��ܼ�������Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 __________________________________________________
__________________________________________________ ______________________��
______________________�� 1)����Һ����ȡ20.00 mL FeSO4��Һ������ƿ�У���0.10 mol��L��1������KMnO4��Һ�����յ㣬��ȥKMnO4��Һ20.00 mL������������MnԪ��ȫ���ʣ�2�ۣ��ζ���Ӧ�����ӷ���ʽΪ______ __���ݴ˿ɲ��FeSO4��Һ�����ʵ���Ũ��Ϊ______ __mol��L��1��
1)����Һ����ȡ20.00 mL FeSO4��Һ������ƿ�У���0.10 mol��L��1������KMnO4��Һ�����յ㣬��ȥKMnO4��Һ20.00 mL������������MnԪ��ȫ���ʣ�2�ۣ��ζ���Ӧ�����ӷ���ʽΪ______ __���ݴ˿ɲ��FeSO4��Һ�����ʵ���Ũ��Ϊ______ __mol��L��1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A������500 mLij���ʵ���Ũ�ȵ���Һ��������ֻ250 mL������ƿ |
| B������������������л��е�NaCl���� |
| C������ʽ�ζ�����ȡ20.00mL���������Һ |
| D����C2H5Cl��NaOH��Һ���ȵĻ�����У�����AgNO3��Һ���ɼ�����Ԫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
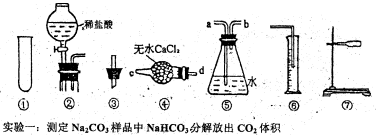
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com