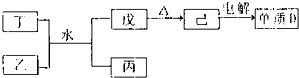
����������Ԫ��A��B��C��D��E��F��ԭ�����������������ǵ�ԭ�Ӻ�����Ӳ���֮��Ϊ13����֪BԪ���γɵĻ�����������࣬DԪ��ԭ�������������Ǵ�����������3����Ԫ��E��ԭ�Ӱ뾶��ͬ���������FԪ��ԭ��������������������Ӳ�����
��1��CԪ����Ԫ�����ڱ��е�λ����
�ڶ����ڵڢ�A��
�ڶ����ڵڢ�A��
��B�ĵ�����C������������Ӧˮ�����Ũ��Һ������Ӧ�Ļ�ѧ����ʽ��
C+4HNO
3��Ũ��
CO
2��+4NO
2��+2H
2O
C+4HNO
3��Ũ��
CO
2��+4NO
2��+2H
2O
��2����A��B��D��E����Ԫ���γɵĻ������������Ļ�ѧ��������
���Ӽ������ۼ�
���Ӽ������ۼ�
��3���E
2D
2��A
2D��Ӧ�����ӷ���ʽ
2Na2O2+2H2O=4Na++4OH-+O2��
2Na2O2+2H2O=4Na++4OH-+O2��
��
��4��ʵ��������100mL0.2mol?L
-1��A��D��E�γɻ��������Һ����Ҫ�õ��IJ����������ձ���С�ձ����
100mL����ƿ������������ͷ�ι�
100mL����ƿ������������ͷ�ι�
���������ʱ���ӿ̶��ߣ�����������ҺŨ��
��
��
0.2mol?L
-1��������������������=��������F�ĵ��������������Ƶ���Һ����������Ӧ�����ӷ���ʽ��
2Al+2OH-+2H2O=+2AlO2-+3H2��
2Al+2OH-+2H2O=+2AlO2-+3H2��
��




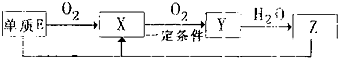
 ����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ����
����������Ԫ��A��B��C��D��E��ԭ������������������ԭ�Ӻ���ĵ��Ӳ���֮��Ϊ10��BԪ�صĻ���������࣬��Ŀ�Ӵ�C��D����Ԫ���γɵĵ����ǿ����к����������ʣ�D��E��Ԫ�ؿ����������ֲ�ͬ�����ӻ���� NH3?H2O+H+
NH3?H2O+H+ NH3?H2O+H+
NH3?H2O+H+ 2NO2
2NO2 2NO2
2NO2