
����Ŀ��Ϊ��ȥ�����е�Ca2+. Mg2+. SO42-�Լ���ɳ�����ʣ�ijͬѧ�����һ���Ʊ����ε�ʵ�鷽�����������£����ڳ������Լ��Թ�����
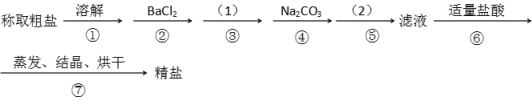
��1���ڢܲ��У�д����Ӧ�����ӷ���ʽ���������Һ��Ca2+����Ҫ������ʽΪCaCl2��_______________ ��____________��
��2��ʵ�鷽���ģ�1����Ӧʹ�ó����Լ��Ļ�ѧʽ__________�����������ӷ���ʽ��__________����ʵ�鷽���ģ�2���еIJ���������_______��
��3����ʵ����Ʒ����Ż��ĽǶȷ�������ںܿ͢ɷ�ߵ�____________����ǡ������������˵�����ɡ�___________________________________________��
��4���ж�BaCl2�ѹ����ķ�����_________________________________________________��
���𰸡�Ca2�� + CO32�� = CaCO3�� Ba2��+ CO32�� = BaCO3�� NaOH 2OH-+Mg2+=Mg(OH)2�� ���� �� ������BaCl2����Ҫ��Na2CO3��ȥ ����ȡ�ϲ���Һ�����μ�BaCl2��Һ�����������ɣ���BaCl2�����������𰸺������ɣ�
��������
��1��Ca2+��CO32-��Ӧ����CaCO3������Ba2��CO32-��Ӧ����BaCO3������
��2���������ƿ��Խ���Һ�е�þ���ӳ�����
��3��̼���Ʊ�������Ȼ����ĺ������,���ܵߵ����������ƺ��Ȼ����ļ�����˳��Ҫ��
��4���Ȼ�������ʱ,��Һ�в��Ậ�����������,���Լ����Ƿ��������������ȷ���Ȼ����Ƿ������
��1��̼���Ƶ������ǽ���Һ�еĸ����Ӻ����ı����ӳ�������,������Ӧ�����ӷ���ʽ�ֱ�Ϊ��Ca2�� + CO32�� = CaCO3����Ba2��+ CO32�� = BaCO3����
�������Ca2�� + CO32�� = CaCO3����Ba2��+ CO32�� = BaCO3����
��2���������ƿ�����Һ�е�þ�����γ�Mg(OH)2������Ȼ������Գ�ȥMg2+�����ӷ���ʽΪ��2OH-+Mg2+=Mg(OH)2����
�����Ϊ��NaOH��2OH-+Mg2+=Mg(OH)2�������ˡ�
��3��̼���Ʊ�������Ȼ����ĺ������,����̼���Ƽȿ��Խ��������Ӹ����ӳ�ȥ,�ֿ��Խ������ı����ӳ�ȥ,����Ba2+��������Ӱ�쾫�εĴ��ȣ��ʲ��ܵߵ��������Ϊ��������BaCl2����Ҫ��Na2CO3��ȥ��
��4���Ȼ�������ʱ����Һ�в��Ậ����������ӣ����Լ�����Һ���Ƿ��������������ȷ���Ȼ����Ƿ����������������:ȡ���ú���ϲ���Һ���ڵ�ΰ���(��ȡ�����ϲ���Һ���Թ���)���ٵ���1~2��BaCl2��Һ,����Һδ�����,�����BaCl2�ѹ�����������ȷ����: ����ȡ�ϲ���Һ�����μ�BaCl2��Һ�����������ɣ���BaCl2������


 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ˮ����ȡ���д��������Һ����Ҫ�����Һ��HClO���ʵ���Ũ�ȣ���Ҫ������Һ��HClŨ�ȣ����д�ʩ���Բ��õ���(����)
A. ���Ȼӷ�HCl B. ��CaSO3
C. ��NaOH�к�HCl D. ��CaCO3�к�HCl
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����( )
A. �ڳ��¡���ѹ�£�11.2 L N2���еķ�����Ϊ0.5NA
B. ��״���£�22.4 L H2��O2�Ļ����������������ΪNA
C. 18 g H2O�����ʵ�����1mol
D. ����£�1 mol SO2�������22.4 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ҩ��F���п���������Ѫ�ǡ���Ѫѹ�ȶ���������ԣ���ϳ�·�����£�
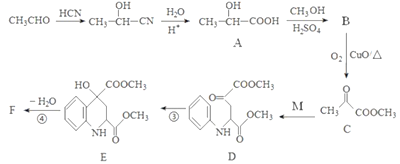
��֪��M�Ľṹ��ʽΪ�� ��
��
��ش��������⣺
(1)A�Ļ�ѧ������______________________��
(2)C�й����ŵ�������_________________________��
(3)д��F�Ľṹ��ʽ____________________________��
(4)��֪A��һ�������������ɿɽ���ľ�������д���÷�Ӧ��ѧ����ʽ��_________________��
(5)��������������M��ͬ���칹����_____��(���������칹)��
���ܹ�����������Ӧ��
�ں�������(-NO2)��������ֱ�����ڱ����ϡ�
�ۺ��б����ұ�����ֻ������ȡ������
���к˴Ź�������Ϊ������ҷ����֮��Ϊ6��2��2��1�Ľṹ��ʽΪ______________(д��һ�ּ���)��
(6)д������ȩΪԭ���Ʊ��߷��ӻ�����۱�ϩ��ĺϳ�·��(���Լ���ѡ)��_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ijԪ�ص�ԭ�Ӻ��ڵ�������Ϊm��������Ϊn���������۶���ȷ���ǣ� ��
A. �����ɴ�ȷ����Ԫ�ص����ԭ������
B. ����Ԫ�ص����ԭ������Ϊ(m+n)g
C. ̼ԭ������Ϊwg����ԭ�ӵ�����Ϊ(m+n)wg
D. �������ӵ�������С�����ӵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�����÷�������(��Ҫ�ɷ�ΪNi��������һ������Zn��Fe��SiO2��CaO��)�Ʊ�������������������£�
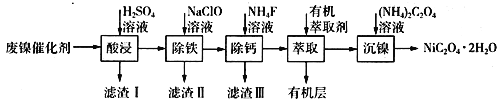
(1)��д��һ������ߡ���������ʵĴ�ʩ��________________________������I�ijɷ���____________(�ѧʽ)��
(2)����ʱ�����Ʋ�ͬ���������Եõ���ͬ������II����֪����II�ijɷ����¶ȡ�pH�Ĺ�ϵ��ͼ��ʾ��
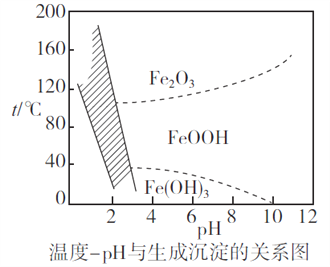
���������¶�40�桢pH=8��������II����Ҫ�ɷ�Ϊ_________________________(�ѧʽ)��
���������¶�80�桢pH=2���ɵõ���������[Na2Fe6(SO4)4(OH)12](ͼ����Ӱ����)��д�����ɻ������Ƶ����ӷ���ʽ��___________________________________________��
(3)��֪����������100 mL��Һ��c(Ca2+)=0.01mol��L-1������100 mL NH4F��Һ��ʹCa2+ǡ�ó�����ȫ����Һ��c(Ca2+)=1��10-5 mol��L-1��������c(NH4F)=_________mol��L-1��[��֪Ksp(CaF2)=5.29��10-9]
(4)�����л���ȡ����������________________________��
(5)ij��ѧ�����Լ��Ļ�ѧʽΪMxNi(SO4)y(MΪ+1�������ӣ�NiΪ+2�ۣ�x��y��Ϊ������)��Ϊ�ⶨ�ö����Լ�����ɣ���������ʵ�飺
I������28.7g�����Լ�������100 mL��ҺA��
��ȷ��ȡ10.00 mL��ҺA����0.40 mol��L-1��EDTA(Na2H2Y)����Һ�ζ����е�Ni2+(���ӷ���ʽΪNi2++H2Y2-=NiY2-+2H+)������EDTA����Һ25.00mL��
����ȡ10.00 mL��ҺA������������BaCl2��Һ���õ���ɫ����4.66g��
������100 mL�����Լ�ʱ����Ҫ��������ҩ�ס�������ƽ�����������ձ�����Ͳ����ͷ�ι��⣬����Ҫ________________________��
�ڸö����Լ��Ļ�ѧʽΪ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����C2H2Ϊԭ�Ϻϳɻ���ϩ�����L��·�����£�
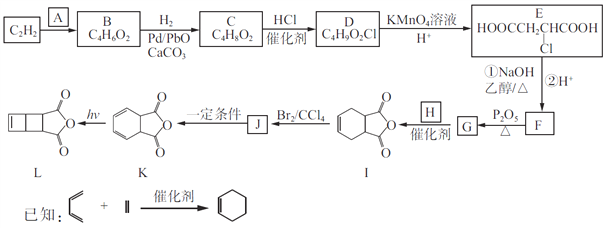
(1)A��̼���⡢������Ԫ����ɣ���Է���������30����A������Ϊ____________��
(2)B�����е�̼ԭ�Ӷ���ͬһֱ���ϣ�B�Ľṹ��ʽΪ________________��
(3)C��D��J��K�ķ�Ӧ���ͷֱ�Ϊ________________��________________��
(4)���C��D��E��F������Ӧ��Ŀ����________________________��
(5)G��H����I�Ļ�ѧ����ʽΪ______________________��
(6)������X��I��ͬ���칹�壬����FeCl3��Һ������ɫ��Ӧ��������NaHCO3��Һ��Ӧ�ų�CO2��X����__��(�����������칹)�����к˴Ź�������Ϊ����壬�������Ϊ2��2��2��1��1�Ľṹ��ʽΪ____________________��
(7)д����![]() ����ȲΪԭ���Ʊ�
����ȲΪԭ���Ʊ�![]() �ĺϳ�·��(�����Լ���ѡ)_____��
�ĺϳ�·��(�����Լ���ѡ)_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʷ����У�ǰ�߰������ߵ��ǣ�������
A.����� ʯī
B.���� ��ɢϵ
C.����� Ư��
D.���������� CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ϴ�Ӻ�SO2���������������ʿ���Ϊϴ�Ӽ�����
A. Ca(OH)2B. CaCl2C. NaHSO3D. HCl
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com