
ͭ���仯�������������������й㷺��Ӧ�á�
(1)ͭ�ɲ������·����Ʊ���
����ͭ��Cu2S��O2 2Cu��SO2
2Cu��SO2
ʪ����ͭ��CuSO4��Fe=FeSO4��Cu
�������ַ����У�ͭԪ�ؾ���________(���������ԭ��)��ͭ���ʡ�
(2)ӡˢ��·����ʹ�õ�ͭ��Ҫ�������á�
����һ����FeCl3��Һ����ӡˢ��·���Ʊ�CuCl2��2H2O��ʵ����ģ����չ������£�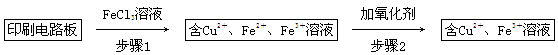

��֤������1����FeCl3��Һ�����ķ�����_________________________________��
�ڲ���2�����ӵ������������˵���______________________________________��
A��HNO3 B��H2O2 C��KMnO4
�۲���3��Ŀ����ʹ��Һ��pH���ߵ�4.2����ʱFe3����ȫ��������ѡ�õġ��Լ�1����________��(д��һ�ּ���)
������Ũ��CuCl2��Һʱ��Ҫ�μ�Ũ���ᣬĿ����________(�û�ѧ����ʽ����ϼ�Ҫ������˵��)���پ���ȴ���ᾧ�����ˣ��õ�CuCl2��2H2O��
����������H2O2��ϡ���Ṳͬ����ӡˢ��·���Ʊ�����ͭʱ�����Ȼ�ѧ����ʽ�ǣ�
Cu(s)��H2O2(l)��H2SO4(aq)=CuSO4(aq)��2H2O(l)����H1����320 kJ��mol��1
��֪��2H2O2(l)=2H2O(l)��O2(g)��H2����196 kJ��mol��1
H2(g)��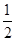 O2(g)=H2O(l)����H3����286 kJ��mol��1
O2(g)=H2O(l)����H3����286 kJ��mol��1
��ӦCu(s)��H2SO4(aq)=CuSO4(aq)��H2(g)�Ħ�H��________��
(3)��ʵ�ַ�ӦCu��H2SO4=CuSO4��H2����������Ϊ��ʵ�ָ�ת����װ���е������ڣ�����缫����(�Cu����C��)��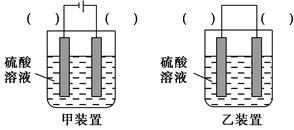

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�����о�С�飬�ú��н϶����ʵ�ͭ�ۣ�ͨ����ͬ�Ļ�ѧ��Ӧ��ȡ����������Ƶ�ʵ�����Ϊ��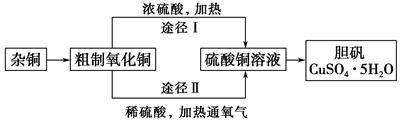
(1)��ͭ�к��д������л���ɲ������յķ�����ȥ�л������ʱ������������ ��(���������������ı�����룬��ͬ)��ȡ������Ӧʹ�� �����պ������Ӧ���� �ϣ�����ֱ�ӷ��������ϡ�
ʵ������������
a������bʯ������c�����ǣ�d������e����ǯ��f�Թܼ�
(2)��ͭ�����պ�õ��IJ���������ͭ������ͭ�Ļ������պ�������ͭ�Ŀ���ԭ���� ��
a�����չ����в�������ͭ����ԭ
b�����ղ����ͭδ����ȫ����
c������ͭ�ڼ��ȹ����зֽ�����ͭ
d����������ͭ������������
(3)ͨ��;����ʵ���ô�������ͭ��ȡ������������е�ʵ��������裺���ܡ�����ͨ���������ˡ� ����ȴ�ᾧ�� ����Ȼ���
(4)�ɴ�������ͭͨ������;����ȡ��������;������ȣ�;���������Ե������ŵ��� �� ��
(5)�ڲⶨ���õ���(CuSO4��xH2O)�нᾧˮxֵ��ʵ������У������������ٽ��� �Ρ�
(6)���ⶨ���xֵƫ�ߣ����ܵ�ԭ���� (����ĸ���)��
a�������¶ȹ���
b����������Ŀ����ϴ�
c�����Ⱥ���ڿ�������ȴ
d���������岿�ַ绯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ɱ�ͭ��mCu2O��nFeS��ұ���õ���ͭ�����Դ�ͭΪԭ���ƴ�ͭ���������£�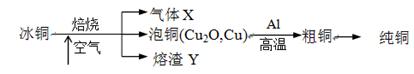
��ش��������⣺
��1������X�� ��
��2��ij�о���ѧϰС��������Y��CO��Ӧ����ȡFe��
���밴���������ҵķ����������и�װ�ã�˳��ΪA��________��
��װ��C��������________________��
���ڵ�ȼD���ľƾ���ǰ��Ӧ���еIJ�����__________________��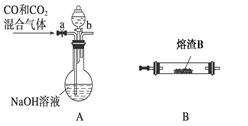
��3������Y����Ԫ�صļ�̬��+2�ۺ�+3�ۣ�������ѡ�Լ������ʵ�鷽����֤����Y����+2����Ԫ�ش��ڣ�д���й�ʵ�������Ԥ����������ۡ�
��ѡ�Լ��� 3 mol��L-1H2SO4��6 mol��L-1HNO3��3�� H2O2��0.01 mol��L-1KMnO4��20�� KSCN��
��
��4��д����ͭұ����ͭ��Ӧ�Ļ�ѧ����ʽ ��
��5��������ѡ���ϻ����ô�ͭ������ͭ��װ��ͼ�������б�Ҫ�ı�ע��
��ѡ���ϣ�FeSO4(aq)��CuSO4(aq)����������������ͭ����ͭ���ձ���ֱ����Դ�����ߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ������£�
(ע��Al(OH)3��Cu(OH)2��ʼ�ֽ���¶ȷֱ�Ϊ450 ���80 �档)
(1)��⾫����ʱ��������ӦʽΪ________������A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���������ɫ�Ļ�ѧ��Ӧ����ʽΪ________________��
(2)��������B�����Ϊ__________�������ɹ���B�Ĺ����У������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ____________��
(3)������չ�����һ����Ӧ�Ļ�ѧ����ʽ��
CuO��____Al2O3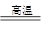 ____CuAlO2��________����
____CuAlO2��________����
(4)����ͭ�Ͻ���ͭ����������Ϊ63.5%��������5.0 kg�����е�ͭ����ȫת��Ϊ________ mol CuAlO2��������Ҫ1.0 mol��L��1��Al2(SO4)3��Һ________ L��
(5)CuSO4��ҺҲ�������Ʊ������������������________�����ˡ�ϴ�Ӻ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ��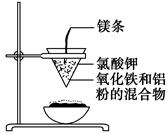
������Բ����ֽ�ֱ��۵���©��״������һ��ʹ���ܶ����IJ㡣���ڲ���ֽȡ�����ڵײ���һ��С�ף���ˮ��ʪ���ٸ���һֽ©������һ�𣬼�����Ȧ�ϣ��������һʢɳ��������5 g�������������ĩ��2 g���ۻ�Ͼ��ȣ�����ֽ©���У��������������ز��ڻ�����м��һ��þ������Сľ����ȼþ�����۲췢��������ͨ������ʵ����Ϣ���ش��������⣺
(1)д���÷�Ӧ�Ļ�ѧ����ʽ��__________��
�ڸ÷�Ӧ��________����������________�ǻ�ԭ�����÷�Ӧ��Ϊ________��Ӧ��
(2)ѡ����ʵ���ʵ������(��д�ں�����)��________��
��þ������ȼ�գ��ڷų��������ȣ�������ҫ�۵Ĺ�â���������䣻��ֽ©�����²����մ������к���״̬��Һ�������������ڵ�ϸɳ�ϣ�Һ����ȴ���Ϊ��ɫ���塣
(3)д�����в��������ʵ����ã��ڲ�ֽ©���ײ���һ���ף�________��������ʢɳ��________��þ����________������أ�________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ����ͭ������ˮ�����ȵ�210������������Cu(NO3)2 ��3H2O����Ļ�ѧ�����нϴ����, Cu(NO3)2 ��3H2O������ȵ�170��ֽ⡣��֪:���������ķе�Ϊ77 �档
��1����������Cu(NO3)2 ��Һ�ò�����ˮ����ͭ��ԭ����_____________��
��2����ͭƬ���˵�N2 O 4������������Һ�п��Ƶ���ˮ����ͭ��ͬʱ����NO,д����Ӧ�Ļ�ѧ����ʽ_____________���������Ҵ��з������ˮ����ͭ��ʵ�������_____________��
��3��Ϊ̽��Cu(NO3)2 ��3H2O���ȷֽ�IJ��ij̽��С��������ͼװ�ý���ʵ�顣(ͼ�мгֺͼ���װ��ȥ)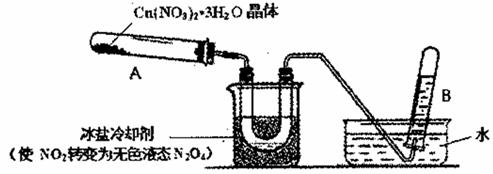
���Թ�A�м�����ϸ��Cu(NO3)2 ��3H2O�岢���ȣ��۲쵽�Թ�A���к���ɫ�����ɣ����ղ�����ɫ��ĩ��U������Һ�����ɣ����Թ�B���ռ�����ɫ���塣
�ٵ����ܿڲ���������ð��ʱ����Ӧֹͣ�����װ�õIJ���������______��
���Թ�B���ռ���������һ������______��
��4��п��Cu(NO3)2��Һ�ܷ�����Ӧ����һ֧�Թ���ע��1 mol��L-1��Cu(NO3)2��Һ���ٷ���һ��пƬ���۲쵽�ڷ�Ӧ�����д�����ɫ����ð����ͬʱпƬ��������ɫ���塣��С���������ijɷ֣�����Ƶ�ʵ�鲽�裬����д�±���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��Q��R�����ֶ�����Ԫ�أ�ԭ��������������X��Y��Ԫ����������������֮�;�Ϊ0��Q��Xͬ���壻Z��R�ֱ��ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�ء�
��ش��������⣺
(1)����Ԫ��ԭ�Ӱ뾶�ɴ�С��˳����(дԪ�ط���)________________��
(2)X��Y���γɶ��ֻ�������мȺ����Լ��ֺ��Ǽ��Լ�������Է���������С��������(д����ʽ)____________��
(3)������ijЩԪ����ɵĻ�����A��B��C��D������ת����ϵ��
A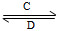 B(��ˮ��Һ�н���)
B(��ˮ��Һ�н���)
���У�C������ˮ�����Ե����壻D�ǵ���ɫ���塣д��C�Ľṹʽ��________��D�ĵ���ʽ��________��
�����A��B��������Ԫ����ɣ�BΪ���Բ������A�Ļ�ѧʽΪ________����Aת��ΪB�����ӷ���ʽΪ______________________________��
�����A������Ԫ����ɣ�B������Ԫ����ɣ�A��B��Һ���Լ��ԡ������ӷ���ʽ��ʾA��Һ�Լ��Ե�ԭ��______________________��A��BŨ�Ⱦ�Ϊ0.1 mol��L��1�Ļ����Һ�У�����Ũ���ɴ�С��˳����________�������£��ڸ���Һ�еμ�ϡ����������ʱ�����ʵ���Ҫ�ɷ���______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͭ�ڹ�ũҵ�����������ж�����Ҫ��Ӧ�á���ش��������⣺
��1��ͭ��Ũ���Ṳ�����Ʊ�����ͭ�ķ���֮һ��д����Ӧ�Ļ�ѧ����ʽ��__________________�����÷�Ӧ���ɵ�������Ⱦ������Ϊ�������Ⱦ������ͭ����ϡ����Ļ������ͨ���ȿ�������Ӧ���ܻ�ѧ����ʽΪ____________________��
��2����ͭ������ϡ�����в�������Ӧ������˫��ˮ����ͭ�ۿ����ܽ⡣д����Ӧ�����ӷ���ʽ��____________________��
��3��������ϡ����ֶ�μ��뵽ͭ����ϡ����Ļ�����У�����ʹ֮��Ӧ��ȫ��ͨ���������ᾧ�ɵõ�����ͭ���塣Ϊ�˽�Լԭ�ϣ�H2SO4��HNO3�����ʵ���֮�����Ϊ________________��
��4���ö��Ե缫�������ͭ��Һ��ʵ��װ����ͼ����ʾ��ͼ���ǵ������в�������������V��ת�Ƶ��ӵ����ʵ���n��e�����Ĺ�ϵͼ��
�������У�a�缫��������______________��b�缫�ĵ缫��ӦʽΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O),��Ҫ�����������¡�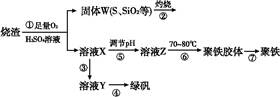
(1)�����̢ڲ���������ͨ��������Һ��,��Һ����ɫ������������
A.Ʒ����Һ B.��ɫʯ����Һ
C.����KMnO4��Һ D.��ˮ
(2)���̢���,FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ ��
(3)���̢����������������� ��
(4)���̢���,�����ᾧʱ��ʹ�õ��������ƾ��ơ����ż���,����Ҫ ��
(5)���̢ݵ���pH��ѡ�������Լ��е���������(�����)��
A.ϡ���� B.CaCO3 C.NaOH��Һ
(6)���̢���,����ҺZ���ȵ�70��80 ��,Ŀ������ ��
(7)ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص���������,��������ʵ�顣���÷�����ƽ��ȡ2.700 0 g��Ʒ;�ڽ���Ʒ���������������,���������BaCl2��Һ;�۹��ˡ�ϴ�ӡ�����,���صù�������Ϊ3.495 0 g�����þ�������Ҫ�ɷ�Ϊ[Fe(OH)(SO4)]n,��þ�����Ʒ����Ԫ�ص���������Ϊ����������(���������в�����Ԫ�غ���Ԫ��)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com