
��ͼ��ʾ��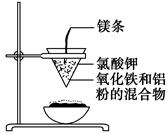
������Բ����ֽ�ֱ��۵���©��״������һ��ʹ���ܶ����IJ㡣���ڲ���ֽȡ�����ڵײ���һ��С�ף���ˮ��ʪ���ٸ���һֽ©������һ�𣬼�����Ȧ�ϣ��������һʢɳ��������5 g�������������ĩ��2 g���ۻ�Ͼ��ȣ�����ֽ©���У��������������ز��ڻ�����м��һ��þ������Сľ����ȼþ�����۲췢��������ͨ������ʵ����Ϣ���ش��������⣺
(1)д���÷�Ӧ�Ļ�ѧ����ʽ��__________��
�ڸ÷�Ӧ��________����������________�ǻ�ԭ�����÷�Ӧ��Ϊ________��Ӧ��
(2)ѡ����ʵ���ʵ������(��д�ں�����)��________��
��þ������ȼ�գ��ڷų��������ȣ�������ҫ�۵Ĺ�â���������䣻��ֽ©�����²����մ������к���״̬��Һ�������������ڵ�ϸɳ�ϣ�Һ����ȴ���Ϊ��ɫ���塣
(3)д�����в��������ʵ����ã��ڲ�ֽ©���ײ���һ���ף�________��������ʢɳ��________��þ����________������أ�________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����(Na2CO3)�����������о��й㷺����;��������ʵ����ģ���Ƽ�ԭ����ȡNa2CO3������ͼ��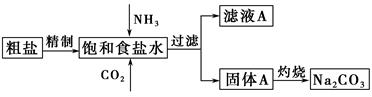
��֪����ʳ��ˮ��ͨ��NH3��CO2�����ķ�ӦΪNaCl��NH3��CO2��H2O=NaHCO3����NH4Cl����ش��������⣺
(1)�����к��е�����������Ca2����Mg2����SO42���ȡ�
���Ƴ��ӵIJ���˳����a�� �� �� ��b(����ĸ���)��
a�������ܽ⣬��ȥ����
b�����������pH
c������Ba(OH)2��Һ
d������Na2CO3��Һ
e������
��ʳ��ˮ����ͨ��NH3����ͨ��CO2�������� ��
(2)���չ���A��Na2CO3�� (����ĸ���)�н��С�
a������ b�������� c���ձ� d����ƿ
֤����ҺA�к���NH4���ķ����� ��
����ҺA�����ؽᾧ�ܹ����NH4HCO3����pH��13��Na����K������Һ�м�������NH4HCO3ʹpH���ͣ���Ӧ�����ӷ���ʽΪ ��
(3)��ͼװ���г�����ʵ�����Ʊ�CO2���� (����ĸ���)����bװ���Ʊ�NH3����Һ©����ʢ�ŵ��Լ��� (���Լ�����)����ƿ�ڿɼ���Ĺ����Լ��� (���Լ�����)��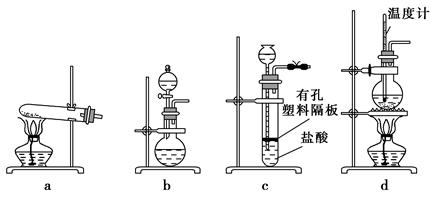
(4)һ����Ȼ���ɷ���aNa2CO3��bNa2SO4��cH2O��ijͬѧ���������ṩ���Լ�����������¼����ⶨNa2CO3������������ʵ�鷽����(������ѡ)���ʵ�鷽����ȫ��
��ѡ����Լ���1.0 mol��L��1 H2SO4��Һ��1.0 mol��L��1 BaCl2��Һ��ϡ��ˮ����ʯ�ҡ�Ca(OH)2��Һ������ˮ
�ٳ�ȡm1g��Ȼ�����Ʒ��������������ˮ�С�
�� ��
�� ��
�ܼ�����Ȼ����к�Na2CO3������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ӧ����㷺�Ľ���������±����������Լ������ĺ������ξ�Ϊ��Ҫ�����
(1)Ҫȷ������ij�Ȼ���FeClx�Ļ�ѧʽ���������ӽ����͵ζ��ķ�����ʵ���г�ȡ0.54 g��FeClx��Ʒ���ܽ���Ƚ��������ӽ���Ԥ��������ͨ�����б���OH���������ӽ�������ʹCl����OH������������������ɺ�������Һ��OH����0.40 mol��L��1������ζ��������յ�ʱ��������25.0 mL���������Ʒ���ȵ����ʵ����������FeClx��xֵ��______________________________________ (�г��������)��
(2)����һ����FeCl2��FeCl3�Ļ������Ʒ�����������������n(Fe)��n(Cl)��1��2.1�������Ʒ��FeCl3�����ʵ�������Ϊ________����ʵ�����У�FeCl2�������ۺ�________��Ӧ�Ʊ���FeCl3�������ۺ�________��Ӧ�Ʊ���
(3)FeCl3������ᷴӦʱ��������ɫ���ʣ��÷�Ӧ�����ӷ���ʽΪ________________��
(4)�������(K2FeO4)��һ��ǿ������������Ϊˮ��������������ز��ϡ�FeCl3��KClO��ǿ���������·�Ӧ����ȡK2FeO4���䷴Ӧ�����ӷ���ʽΪ_____________________________����MnO2��Zn������ƣ�K2FeO4��ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ�������ϣ���缫��ӦʽΪ______________________���õ���ܷ�Ӧ�����ӷ���ʽΪ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D����ѧ��ѧ�������������ʣ����ǵ�ת����ϵ���£� A + B =" C" + D
��1����A��B��C���Ƿǽ����������C�ڿ������ױ��A����A�з���Ԫ�ص���̬�⻯��M�ĵ���
ʽΪ �����ӵ��ȶ���B M���>������<����=����
��2����A��D�����ֵ��ʣ�B�Ǻ�ɫ�д��Ե������B�����ᷴӦ������ҺN������N��Һ�еͼ۽��������ӵķ����� ��
��D�ķ�ĩ������ҺN�У�����28gD��ȫ�ܽ�ʱ���÷�Ӧת�Ƶĵ����� mol��
��3����A��B��C��D������Ӧ��2CO2(g) + 6H2(g) = CH3CH2OH(g) + 3H2O(g)��������ͼ��ʾ��Ϣ��
�ٷ�Ӧ�� ��Ӧ������ȡ������ȡ������ж�������
������H =" a" KJ/mol�������ı����5.6 L CO2ʱ�ķ�Ӧ�ȡ�H = KJ/mol��
���ں��¡����ݵ��ܱ������У�
����������˵��������Ӧ�Ѵﻯѧƽ��״̬���� ������ĸ��ţ���
A������1 mol CH3CH2OH��ͬʱ������3 mol H2O
B�������и���ݵ����ʵ���Ũ�Ȳ���ʱ����仯
C�������л��������ܶȲ���ʱ����仯
D������������ķ�����������ʱ����仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��X��Ϊ��ѧ�����Ĵ��������֮������ͼת����ϵ(����������ȥ)��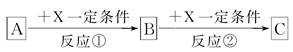
�ش��������⣺
(1)��X��ǿ�����Ե��ʣ���A��������________��
a��S b��N2 c��Na d��Mg e��Al
(2)��A��B��CΪ������Ԫ�ص��������XΪǿ�������Һ��A��Һ��C��Һ��Ӧ����B����B�Ļ�ѧʽΪ________��X�Ļ�ѧʽ����Ϊ(д����ͬ������)________��________����Ӧ�ٵ����ӷ���ʽΪ________________________��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͭ���仯�������������������й㷺��Ӧ�á�
(1)ͭ�ɲ������·����Ʊ���
����ͭ��Cu2S��O2 2Cu��SO2
2Cu��SO2
ʪ����ͭ��CuSO4��Fe=FeSO4��Cu
�������ַ����У�ͭԪ�ؾ���________(���������ԭ��)��ͭ���ʡ�
(2)ӡˢ��·����ʹ�õ�ͭ��Ҫ�������á�
����һ����FeCl3��Һ����ӡˢ��·���Ʊ�CuCl2��2H2O��ʵ����ģ����չ������£�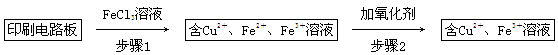

��֤������1����FeCl3��Һ�����ķ�����_________________________________��
�ڲ���2�����ӵ������������˵���______________________________________��
A��HNO3 B��H2O2 C��KMnO4
�۲���3��Ŀ����ʹ��Һ��pH���ߵ�4.2����ʱFe3����ȫ��������ѡ�õġ��Լ�1����________��(д��һ�ּ���)
������Ũ��CuCl2��Һʱ��Ҫ�μ�Ũ���ᣬĿ����________(�û�ѧ����ʽ����ϼ�Ҫ������˵��)���پ���ȴ���ᾧ�����ˣ��õ�CuCl2��2H2O��
����������H2O2��ϡ���Ṳͬ����ӡˢ��·���Ʊ�����ͭʱ�����Ȼ�ѧ����ʽ�ǣ�
Cu(s)��H2O2(l)��H2SO4(aq)=CuSO4(aq)��2H2O(l)����H1����320 kJ��mol��1
��֪��2H2O2(l)=2H2O(l)��O2(g)��H2����196 kJ��mol��1
H2(g)��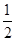 O2(g)=H2O(l)����H3����286 kJ��mol��1
O2(g)=H2O(l)����H3����286 kJ��mol��1
��ӦCu(s)��H2SO4(aq)=CuSO4(aq)��H2(g)�Ħ�H��________��
(3)��ʵ�ַ�ӦCu��H2SO4=CuSO4��H2����������Ϊ��ʵ�ָ�ת����װ���е������ڣ�����缫����(�Cu����C��)��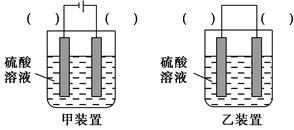
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ����ȡ�طʺ�������������Ҫԭ�ϣ�����ʯ����ɺ��������ƣ�����������������������������ʡ�����ʵ�鲽������ͼ��ʾ��
��������ͼʾ�����������գ�
��1������ʯ���պ���ϡ��ˮ����������500mLϡ��ˮ��ÿ������39.20g������ҪȡŨ��ˮ��ÿ������251.28g����__________mL���ù��Ϊ_______mL��Ͳ��ȡ��
��2����ˮ������õ���������ϵ�����ˣ���Һ�г�K����SO42���⣬���д�����NH4��������NH4���ķ�����______________________________________________��
��3��д�����������������ʵĻ�ѧʽ________________________________________��
��4����ҺI�ijɷ���ˮ��______________��
��5��Ϊ�ⶨ��Ϸ���K2SO4��(NH4)2SO4�мصĺ��������������в��裺
�ٳ�ȡ�ص�������������ˮ����������______��Һ��������ɫ������
��___________��__________��_________(������дʵ���������)��
����ȴ�����ء�
��6��������Ϊmg�����������ʵ���Ϊnmol����������K2SO4�����ʵ���Ϊ��___________mol���ú���m��n�Ĵ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E����ѧ�γ�����5�ֻ����A��B�������Ԫ��X��Y�ĵ����������г����Ľ�����������ʼ��ת����ϵ��ͼ��ʾ��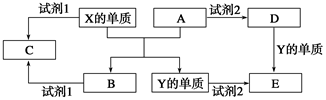
(1)X�ĵ�����A��Ӧ�Ļ�ѧ����ʽ��_______________________________��
(2)���Լ�1��NaOH��Һ����X�ĵ������Լ�1��Ӧ�����ӷ���ʽ��
___________________________________
(3)���Լ�1���Լ�2����ϡ���ᡣ
�ټ�������D����Һ�н������ӵķ�����___________________________________��
�ڽ�����C����ˮ������Һ�����ԣ�ԭ����(�����ӷ���ʽ��ʾ)
________________________________________________��
��ij��Ч��ˮ������Y(OH)SO4�ۺϵõ��ģ���ҵ����E��ϡ�������������Ϊԭ���Ʊ�Y(OH)SO4����Ӧ����NO���ɣ��÷�Ӧ�Ļ�ѧ����ʽ��_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͭ��ʯ��Ҫ��������ͭ��Cu2S����������ʯ��SiO2����һ���Ի�ͭ��ʯΪԭ���Ʊ�����ͭ�Ĺ����������£�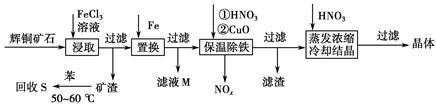
��1��д����ȡ������Cu2S�ܽ�����ӷ���ʽ___________________________��
��2������S�������¶ȿ�����50��60 ��֮�䣬���˹�����͵�ԭ����____________________��____________________��
��3������NOx��������Ϻ�ͨ��ˮ�������������п�ѭ�����õ�һ�����ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ______________________������ҺM�м��루��ͨ�룩����________������ĸ�����ʣ��õ���һ�ֿ�ѭ�����õ����ʡ�
a������������b��������������c���������
��4�����³��������м���CuO��Ŀ����____________________������Ũ��ʱ��Ҫ�����������Һ��pH����������______________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com