
��ͭ��ʯ��Ҫ��������ͭ��Cu2S����������ʯ��SiO2����һ���Ի�ͭ��ʯΪԭ���Ʊ�����ͭ�Ĺ����������£�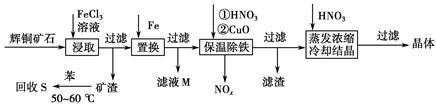
��1��д����ȡ������Cu2S�ܽ�����ӷ���ʽ___________________________��
��2������S�������¶ȿ�����50��60 ��֮�䣬���˹�����͵�ԭ����____________________��____________________��
��3������NOx��������Ϻ�ͨ��ˮ�������������п�ѭ�����õ�һ�����ʣ��÷�Ӧ�Ļ�ѧ����ʽΪ______________________������ҺM�м��루��ͨ�룩����________������ĸ�����ʣ��õ���һ�ֿ�ѭ�����õ����ʡ�
a������������b��������������c���������
��4�����³��������м���CuO��Ŀ����____________________������Ũ��ʱ��Ҫ�����������Һ��pH����������______________________________��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ��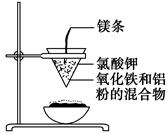
������Բ����ֽ�ֱ��۵���©��״������һ��ʹ���ܶ����IJ㡣���ڲ���ֽȡ�����ڵײ���һ��С�ף���ˮ��ʪ���ٸ���һֽ©������һ�𣬼�����Ȧ�ϣ��������һʢɳ��������5 g�������������ĩ��2 g���ۻ�Ͼ��ȣ�����ֽ©���У��������������ز��ڻ�����м��һ��þ������Сľ����ȼþ�����۲췢��������ͨ������ʵ����Ϣ���ش��������⣺
(1)д���÷�Ӧ�Ļ�ѧ����ʽ��__________��
�ڸ÷�Ӧ��________����������________�ǻ�ԭ�����÷�Ӧ��Ϊ________��Ӧ��
(2)ѡ����ʵ���ʵ������(��д�ں�����)��________��
��þ������ȼ�գ��ڷų��������ȣ�������ҫ�۵Ĺ�â���������䣻��ֽ©�����²����մ������к���״̬��Һ�������������ڵ�ϸɳ�ϣ�Һ����ȴ���Ϊ��ɫ���塣
(3)д�����в��������ʵ����ã��ڲ�ֽ©���ײ���һ���ף�________��������ʢɳ��________��þ����________������أ�________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��FeCl3��Һʴ��ͭ�������·��Ĺ����У���Һ��������Դ���յĹ��̼������£�
�����Һ��Ͷ�������м����ַ�Ӧ�������������Һ��
������Һ�м���һ����ʯ��ˮ��������ҺpH��ͬʱ���������Ŀ�����
��֪��Ksp[Fe(OH)3]��4.0��10��38
�ش��������⣺
��1��FeCl3ʴ��ͭ����Ӧ�����ӷ���ʽΪ________________________________________��
��2�����̢������м����Ҫ������__________________������õ��������Ҫ�ɷ���________���ӹ����з����ͭ����õķ�����___________________________________��
��3�����̢��з�����Ӧ�Ļ�ѧ����ʽΪ________________________________��
��4�����̢��е�����Һ��pHΪ5����������Ũ��Ϊ__________________________��(��ʽ����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���㷺Ӧ���ڻ�ѧ��ҵ���ճ������С���ҵ����������(Al2O3��3H2O�ĺ���ԼΪ85%,������ҪΪSiO2��Fe2O3��)ұ�����������������¡�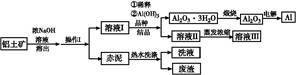
��֪�ݶ�������Al2O3��3H2O�Ļ���ԭ��Ϊ:
Al2O3��3H2O+2NaOH(aq) 2NaAlO2(aq)+4H2O,[Al2O3��3H2OҲ�ɱ�ʾΪ2 Al(OH)3]
2NaAlO2(aq)+4H2O,[Al2O3��3H2OҲ�ɱ�ʾΪ2 Al(OH)3]
(1)�����������Ϊ��������,�����г���������������,�����е���Ҫ������ ����������
(2)Ϊ�������������ܳ����ʿɲ�ȡ����Ч��ʩΪ ��(��д����)��
(3)�û�ѧƽ�����۽���ϡ����Һ��������Al2O3��3H2O �ᾧ��ԭ�� ��
(4)Ϊ������Al2O3��3H2O,Ҳ������Һ����ͨ�����CO2����,д������Al2O3��3H2O�����ӷ���ʽ: ����
(5)Ϊ�˻��ճ����Һ���ߵ����óɷ�,��ҵ�Ͻ�����ˮϴ�Ӻ��ϴҺ������Һ���ϡ�ͼ�,��ָ������ͼ����һ�����Ƶ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������Ҫ�ɷ���Al2O3���������Ĺ�������ʾ��ͼ���£�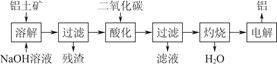
��1��������ɵ���������Һ�� ����ϲ㡱���²㡱�������ʱ�������ĵĵ缫�� ���������������������
��2��д��ͨ�����������̼�ữʱ��Ӧ�����ӷ���ʽ
��
��3������Ʊ���ʱ����������ʯ��Na3AlF6������������ ����ҵ�Ͽ����÷��������塢���������ʹ����ڸ��������·�����Ӧ����ȡ����ʯ��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4������������������������������Fe��Si�����ʣ����õ�ⷽ����һ���ᴿ���õ��ص����������� ���ѧʽ���������ĵ缫��ӦʽΪ ��
��5���Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������
�ٿ���һ���������е�⣨��ͼ������ʱ��������γ��������������Ĥ����缫��ӦʽΪ ��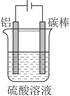
�ڸֲĶ������ܷ�ֹ�ֲĸ�ʴ����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ�����������᳧����(��Ҫ�ɷ�Ϊ���������P����FeS��SiO2��)�Ʊ�����(��ʽ�������ľۺ���)���̷�(FeSO4��7H2O),��Ҫ�����������¡�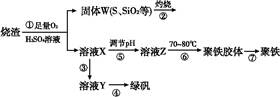
(1)�����̢ڲ���������ͨ��������Һ��,��Һ����ɫ������������
A.Ʒ����Һ B.��ɫʯ����Һ
C.����KMnO4��Һ D.��ˮ
(2)���̢���,FeS��O2��H2SO4��Ӧ�Ļ�ѧ����ʽΪ ��
(3)���̢����������������� ��
(4)���̢���,�����ᾧʱ��ʹ�õ��������ƾ��ơ����ż���,����Ҫ ��
(5)���̢ݵ���pH��ѡ�������Լ��е���������(�����)��
A.ϡ���� B.CaCO3 C.NaOH��Һ
(6)���̢���,����ҺZ���ȵ�70��80 ��,Ŀ������ ��
(7)ʵ����Ϊ�������õ��ľ�����Ʒ����Ԫ�ص���������,��������ʵ�顣���÷�����ƽ��ȡ2.700 0 g��Ʒ;�ڽ���Ʒ���������������,���������BaCl2��Һ;�۹��ˡ�ϴ�ӡ�����,���صù�������Ϊ3.495 0 g�����þ�������Ҫ�ɷ�Ϊ[Fe(OH)(SO4)]n,��þ�����Ʒ����Ԫ�ص���������Ϊ����������(���������в�����Ԫ�غ���Ԫ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧʵ��̽�������Ļ�ԭ�Բ�����ϵ��ʵ�顣
��1�����������Ϻ�ɫͭ˿�Ƽ�Ȧ���ھƾ��ƻ����ϼ��ȣ���ͭ˿��ں��Ƚ�ͭ˿�������ɵ��Ȼ�茶�������̷����а�ɫ�������ɣ��ó�ͭ˿��ͭ˿��ת��Ϊ�������Ϻ�ɫ���ں���ͭ��ʱ���Ȼ�麟�ȥͭ�����������ͭ�������˸�ԭ��������ɸ÷�Ӧ��
��2����������װ�ã����������������MxOy��Ӧ����M��H2��H2O��ͨ����������ˮ���������ⶨM�����ԭ��������a���Լ���Ũ��ˮ��
������a������Ϊ__________������b��װ�˵��Լ�������____________.
�ڰ�����������ȷ��װ������˳��Ϊ������ţ�װ�ÿ��ظ�ʹ�ã���___________��
��ʵ�����ʱ��Ӧ����__________������ţ���
I��Ϩ��Aװ�õľƾ���
II��ֹͣ��a�еμ�Һ��
����ʵ����ȷ��ȡ���������������Ϊmg����ȫ��Ӧ�������ˮ������Ϊng����M�����ԭ������Ϊ__________(�ú�X��y��m��n��ʽ�ӱ�ʾ)��
��3��������������MxOyΪFe2O3������Ӧ���������ϡ���ᣬȻ��μ�KSCN��Һû�������Ա仯���Ʋ�ù���ɷֿ����ǣ�����ѧʽ�����±�������Ϊ�м��ֿ�����֣���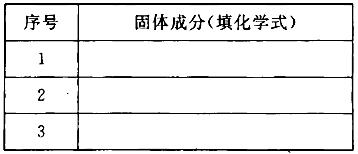
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij����С���һЩ�������ʺͻ���������ʽ���̽����
(1)�±�Ϊ�������Ȼ�ͭ��Һ��Ӧ��ʵ�鱨���һ���֣�
| ʵ�鲽�� | ʵ������ |
| ����ĥ������Ƭ(����)����һ��Ũ�ȵ�CuCl2��Һ�� | �������ݣ��������ɵĺ�ɫ���壬��Һ��Ϊ��ɫ |
| ��Ӧ������������Һ���� | |
| ��ɫ����������ˮϴ�Ӻ����ڳ�ʪ������ | һ��ʱ�������ɺ�ɫ��Ϊ��ɫ[������Ҫ�ɷ�ΪCu2(OH)2CO3] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ɫ������A������ɫ���ߵ�ѹ���������ص㣬�����������˵绯ѧ��ĸ߶����ӡ��ڳ��º���������£�������A�����ȶ��Ĵ��ڣ�������ˮ��Һ�в��ȶ���һ��ʱ���ת��Ϊ���ɫ������ͬʱ����һ�����嵥�ʡ�ij��ȤС���ͬѧ�Ի�����A������ɷ�����ȷ��A�н�����O��K��Fe����Ԫ�ء�ȡ3.96g������A�ķ�ĩ����ˮ���μ�������ϡ���ᣬ��Ӧ�����Һ�м��뺬��0.08mol KOH����Һ��ǡ����ȫ��Ӧ�����ˣ���ϴ�Ӻ�ij���������գ��õ�����ɫ�����ĩ1.60g����������Һ��һ�������������ɵõ�һ�ִ����IJ����ᾧˮ����10.44g��
��1��������A�Ļ�ѧʽΪ ��������A��H2O��Ӧ�����ӷ���ʽΪ ��
��2��������A������Ϊһ�֡���ɫ��Ч��ܡ�ˮ��������ԭ���� ��
��3��������A���Ʊ�����ͨ������������д����KOH�����������ô�������������������Ʊ�A�Ļ�ѧ����ʽ ��
��4��Ŀǰ��������Ի�����A���ȶ��Խ����˴�����̽������ȡ����һ���Ľ�չ�������������п����������Aˮ��Һ�ȶ��Ե���
| A���������� | B��KOH | C������ | D��Fe(NO3)3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com