
����Ӧ����㷺�Ľ���������±����������Լ������ĺ������ξ�Ϊ��Ҫ�����
(1)Ҫȷ������ij�Ȼ���FeClx�Ļ�ѧʽ���������ӽ����͵ζ��ķ�����ʵ���г�ȡ0.54 g��FeClx��Ʒ���ܽ���Ƚ��������ӽ���Ԥ��������ͨ�����б���OH���������ӽ�������ʹCl����OH������������������ɺ�������Һ��OH����0.40 mol��L��1������ζ��������յ�ʱ��������25.0 mL���������Ʒ���ȵ����ʵ����������FeClx��xֵ��______________________________________ (�г��������)��
(2)����һ����FeCl2��FeCl3�Ļ������Ʒ�����������������n(Fe)��n(Cl)��1��2.1�������Ʒ��FeCl3�����ʵ�������Ϊ________����ʵ�����У�FeCl2�������ۺ�________��Ӧ�Ʊ���FeCl3�������ۺ�________��Ӧ�Ʊ���
(3)FeCl3������ᷴӦʱ��������ɫ���ʣ��÷�Ӧ�����ӷ���ʽΪ________________��
(4)�������(K2FeO4)��һ��ǿ������������Ϊˮ��������������ز��ϡ�FeCl3��KClO��ǿ���������·�Ӧ����ȡK2FeO4���䷴Ӧ�����ӷ���ʽΪ_____________________________����MnO2��Zn������ƣ�K2FeO4��ZnҲ������ɼ��Ե�أ�K2FeO4�ڵ������Ϊ�������ϣ���缫��ӦʽΪ______________________���õ���ܷ�Ӧ�����ӷ���ʽΪ_______________��
(1)n(Cl)��0.0250 L��0.40 mol��L��1��0.010 mol
0��54 g��0.010 mol��35.5 g��mol��1��0.19 g
n(Fe)��0.19 g/56 g��mol��1��0.0034 mol
n(Fe)��n(Cl)��0.0034��0.010��1��3��x��3
(2)0.10���� ����
(3)2Fe3����2I��=2Fe2����I2(��2Fe3����3I��=2Fe2����I3-)
(4)2Fe(OH)3��3ClO����4OH��=2FeO42-��5H2O��3Cl��
FeO42-��3e����4H2O=Fe(OH)3��5OH��
2FeO42-��8H2O��3Zn=2Fe(OH)3��3Zn(OH)2��4OH��
ע��Fe(OH)3��Zn(OH)2д���������������ʽ����ȷҲ�ɸ���
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͬ������������Һ����ȫ����ʱ����Һ��pH��ͬ��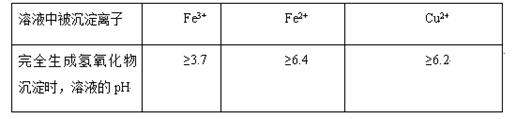
�Ȼ�ͭ���壨CuCl2��2H2O���к�FeCl2���ʣ�Ϊ�Ƶô����Ȼ�ͭ���壬���Ƚ����Ƴ�ˮ��Һ��Ȼ��������ʾ�IJ�����������ᴿ��
��1���������������ʺ���������X���� ������ţ���
| A��NaClO | B��H2O2 | C��KMnO4 | D��Cl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ߡ����������ʸֲĵ�����������ij���������ߡ�����Ҫ�ɷ�Ϊ����M�ͽ�����Ca��������3.5��(��������)CaO��
��1��Ca��ԭ�ӽṹʾ��ͼΪ ��������������Ӧˮ����ļ��Ա�Mg(OH)2 (�ǿ��������)��
��2��Ca��ǽ�������ǿ��Ԫ��A�γɻ�����D���õ���ʽ��ʾD���γɹ��̣� ��
��3����ƽ�á��������ߡ��������ķ���ʽ��
��4�������������ߡ�����ϡ�����,�μ�����˫��ˮ���ٵμ�KSCN��Һ�ʺ�ɫ������MΪ (�ѧʽ)�� ���еμ�˫��ˮ���������ӷ���ʽ�� ��
��5��ȡ3.2 g���������ߡ���������ˮ��ַ�Ӧ����448 mL H2(��״��)����������Һ��ͨ������CO2������ܵõ�CaCO3 g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ȡ�����ʵ�����MgO��Fe2O3�Ļ����������ȷ�Ӧ,��Ӧ�Ļ�ѧ����ʽΪ����������������,�������ȷ�Ӧ��ʵ������������������������ȷ�Ӧʱ,�ڲ�ֽ©���ײ���һС����ˮ��ʪ��Ŀ������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��0.1 mol��þ�������������100 mL 2 mol/L H2SO4��Һ�У�Ȼ���ٵμ�1 mol/L NaOH��Һ����ش�
(1)���ڵμ�NaOH��Һ�Ĺ����У���������m�����NaOH��Һ�����V�仯����ͼ��ʾ����V1��160 mLʱ���������ĩ��n(Mg)��________mol��V2��______________mL��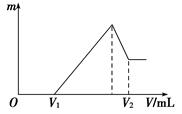
(2)���ڵμ�NaOH��Һ�����У���ʹMg2����Al3���պó�����ȫ�������NaOH��Һ�����V(NaOH)��____________mL��
(3)���������Ϊ0.1 mol������þ�۵����ʵ�������Ϊa����100 mL 2 mol/L�������ܽ�˻������ټ���450 mL 1 mol/L��NaOH��Һ�����ó�������Al(OH)3�������������a��ȡֵ��Χ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ɱ�ͭ��mCu2O��nFeS��ұ���õ���ͭ�����Դ�ͭΪԭ���ƴ�ͭ���������£�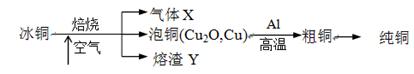
��ش��������⣺
��1������X�� ��
��2��ij�о���ѧϰС��������Y��CO��Ӧ����ȡFe��
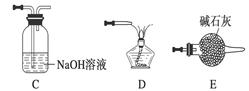
���밴���������ҵķ����������и�װ�ã�˳��ΪA��________��
��װ��C��������________________��
���ڵ�ȼD���ľƾ���ǰ��Ӧ���еIJ�����__________________��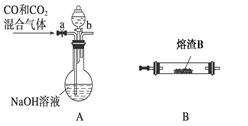
��3������Y����Ԫ�صļ�̬��+2�ۺ�+3�ۣ�������ѡ�Լ������ʵ�鷽����֤����Y����+2����Ԫ�ش��ڣ�д���й�ʵ�������Ԥ����������ۡ�
��ѡ�Լ��� 3 mol��L-1H2SO4��6 mol��L-1HNO3��3�� H2O2��0.01 mol��L-1KMnO4��20�� KSCN��
��
��4��д����ͭұ����ͭ��Ӧ�Ļ�ѧ����ʽ ��
��5��������ѡ���ϻ����ô�ͭ������ͭ��װ��ͼ�������б�Ҫ�ı�ע��
��ѡ���ϣ�FeSO4(aq)��CuSO4(aq)����������������ͭ����ͭ���ձ���ֱ����Դ�����ߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��A��F��Ϊ��ѧ��ѧ�г��������ʣ�����֮��������ת����ϵ������A��D Ϊ�������ʡ�����Ӧ������ˮ��������������ȥ�� 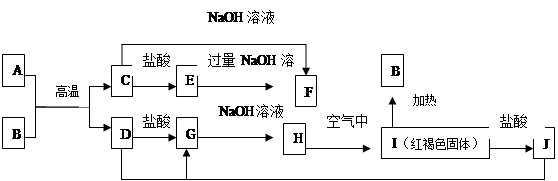
��1��C�� ��J�� ���ѧʽ����
��2��д��C��NaOH��Ӧ����F�Ļ�ѧ����ʽ
��3��A�ڳ�����Ҳ����NaOH��Һ��Ӧ����F��д���˷�Ӧ�Ļ�ѧ��Ӧ����ʽ :
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ��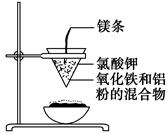
������Բ����ֽ�ֱ��۵���©��״������һ��ʹ���ܶ����IJ㡣���ڲ���ֽȡ�����ڵײ���һ��С�ף���ˮ��ʪ���ٸ���һֽ©������һ�𣬼�����Ȧ�ϣ��������һʢɳ��������5 g�������������ĩ��2 g���ۻ�Ͼ��ȣ�����ֽ©���У��������������ز��ڻ�����м��һ��þ������Сľ����ȼþ�����۲췢��������ͨ������ʵ����Ϣ���ش��������⣺
(1)д���÷�Ӧ�Ļ�ѧ����ʽ��__________��
�ڸ÷�Ӧ��________����������________�ǻ�ԭ�����÷�Ӧ��Ϊ________��Ӧ��
(2)ѡ����ʵ���ʵ������(��д�ں�����)��________��
��þ������ȼ�գ��ڷų��������ȣ�������ҫ�۵Ĺ�â���������䣻��ֽ©�����²����մ������к���״̬��Һ�������������ڵ�ϸɳ�ϣ�Һ����ȴ���Ϊ��ɫ���塣
(3)д�����в��������ʵ����ã��ڲ�ֽ©���ײ���һ���ף�________��������ʢɳ��________��þ����________������أ�________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��FeCl3��Һʴ��ͭ�������·��Ĺ����У���Һ��������Դ���յĹ��̼������£�
�����Һ��Ͷ�������м����ַ�Ӧ�������������Һ��
������Һ�м���һ����ʯ��ˮ��������ҺpH��ͬʱ���������Ŀ�����
��֪��Ksp[Fe(OH)3]��4.0��10��38
�ش��������⣺
��1��FeCl3ʴ��ͭ����Ӧ�����ӷ���ʽΪ________________________________________��
��2�����̢������м����Ҫ������__________________������õ��������Ҫ�ɷ���________���ӹ����з����ͭ����õķ�����___________________________________��
��3�����̢��з�����Ӧ�Ļ�ѧ����ʽΪ________________________________��
��4�����̢��е�����Һ��pHΪ5����������Ũ��Ϊ__________________________��(��ʽ����)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com