
����Ŀ��I.����������Һ���ֱ��������ӣ���Ag+����Mg2+����Fe2+����Al3+����Fe3+��
(1)д�������������������ӷ��������ܱ��������ܱ���ԭ��������______�������ۺ���Һ���ص���____��
(2)��Fe2+����Һ�еμ�NaOH��Һ��������__________________��
(3)��ȥFeCl2��FeCl3�����漰�����ӷ���ʽ��________________��
II.(1)��ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ��ʪ����ɫ�����Ĺ��ƿ���ɹ۲쵽�������ǣ�____________����ʵ��֤����Ư�����õ���______��(�ѧʽ)
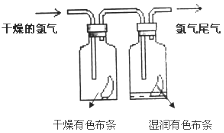
(2)�������ж���ʵ�������ն����������ԭ����(�����ӷ���ʽ��ʾ)__________________��
�ڸ�����һԭ������ҵ�ϳ������۵�ʯ�������չ�ҵ����β���Ƶ�Ư�ۣ�Ư�۵���Ч�ɷ���_________(�ѧʽ)��
�۳���¶���ڿ����е�Ư�ۻ�ʧЧ��ʧЧ��ԭ����(�û�ѧ����ʽ��ʾ)___________________________��________________________��
��Ư���Ƿ���ȫʧЧ����ϡ������飬��ϡ����������������______(����ĸ����)��
A.O2 B.Cl2 C.CO2 D.HClO
���𰸡�Fe2+ Fe3+ ���ɰ�ɫ��״����������Ѹ���ɰ�ɫ��Ϊ����ɫ������Ϊ���ɫ Fe+2Fe3+�T3Fe2+ �������ɫ��������������ʪ����ɫ������ɫ HClO Cl2+2OH-=Cl-+ClO-+H2O Ca(ClO)2 Ca(ClO)2+CO2+H2O=CaCO3��+2HClO 2HClO![]() 2HCl+O2�� C
2HCl+O2�� C
��������
I. (1)�����м��̬�Ľ��������Ӽ��ܱ��������ܱ���ԭ�����ܽ�������Һ���û��������ܺ�Fe3+��Ӧ����Fe2+��
(2) ��Fe2+����Һ�еμ�NaOH��Һ��������Ӧ��Fe2++2OH-=Fe(OH)2������ɫ���������������������ױ���������Ϊ����������
(3) Fe3+�ܱ�����ԭ����Fe2+���Ҳ�������������
II.(1)������Ư���ԣ�������ˮ��Ӧ���ɵĴ��������Ư���ԣ�
(2)��������������������Һ��Ӧ������β����ʯ�������չ�ҵ����β���Ƶ�Ư�������Ȼ��ơ�������ƣ��������ΪƯ�۵���Ч�ɷ֣�����¶���ڿ����е�Ư�ۣ�������Ʊ���Ϊ̼��ƣ���ϡ��������������Ϊ������̼.
I.(1)Fe2+��FeԪ�صĻ��ϼ۴����м��̬�����ܱ��������ܱ���ԭ��������ٵ�Ag+�͢���Fe3+�������ӷ�Ӧ���Ԣٷ�ӦΪ��Fe+2Ag+=2Ag+Fe2+����Һ����������ڢݷ�����ӦΪ��Fe+2Fe3+=3Fe2+����Һ�����������ʼ��ܱ��������ܱ���ԭ��������Fe2+�������ۺ���Һ���ص���Fe3+��
(2) Fe2+������������Һ�е�OH-��Ӧ���ɵ���������������Fe2++2OH-=Fe(OH)2�������������������ױ���������Ϊ����������4Fe(OH)2+O2+2H2O=4Fe(OH)3�����Կ����������ǣ����ְ�ɫ������Ѹ�ٱ�Ϊ����ɫ����Ϊ���ɫ��
(3)��ȥFeCl2��FeCl3ѡ�����ۣ� FeCl3����Fe��Ӧ����FeCl2��Һ�������������ʣ���Ӧ�����ӷ���ʽΪ��Fe+2Fe3+=3Fe2+��
II.(1)������Ư���ԣ�������ˮ��Ӧ���ɵĴ��������Ư���ԣ�Cl2+H2O=HCl+HClO����˻ῴ�����������ɫ��������������ʪ����ɫ������ɫ����ʵ��֤����Ư�����õ���HClO��
(2) ��Ϊ�˷�ֹ����β����Ⱦ����������NaOH��Һ���գ��÷�Ӧ�����ӷ���ʽΪCl2+2OH--=Cl-+ClO-+H2O��
��Cl2��ʯ���鷢����Ӧ��2Cl2+2Ca(OH)2=CaCl2+Ca(ClO)2+2H2O���õ�Ư�ۣ�Ư�۵���Ч�ɷ���Ca(ClO)2��
�۳���¶���ڿ����е�Ư�ۣ���Ч�ɷ�Ca(ClO)2��Ϳ����еĶ�����̼ˮ��Ӧ����̼��ƺʹ����ᣬ����ʽΪ��Ca(ClO)2+CO2+H2O=CaCO3��+2HClO��HClO���ȶ����������ֽ⣬�ֽⷴӦ����ʽΪ��2HClO![]() 2HCl+O2����
2HCl+O2����
��Ư�۳��ڱ�¶�ڿ����У����õ�����Ҫ�ɷ��к�̼��ƣ���ϡ����������ֽⷴӦ��CaCO3+2HCl=CaCl2+H2O+CO2�����ʺ���ѡ����C��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����������װ����ȡ������������������ʵ�顣�ش��������⣺
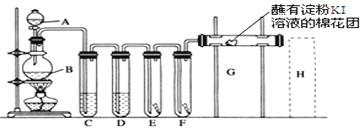
��1��A��ʢ��Ũ���ᣬB��ʢ��MnO2��д����Ӧ�����ӷ���ʽ___________________��
��2��D�з���ŨH2SO4��Ŀ����_____________________________��
��3��E��Ϊ��ɫ�ɲ�����F��Ϊ��ɫʪ�������ɹ۲쵽��������___________���Ա�E��F������IJ���ɵó��Ľ�����________________________________��
��4��G����������____________________________________��
��5���û�ѧ����ʽд��H��β������װ���еķ�Ӧԭ��____________��
��6����ͥ�г�������Һ����Ҫ�ɷ�NaClO�������飨��Ҫ�ɷ����ᣩ���������ijƷ������Һ��װ��˵������ͼ��

������Һ�����鲻��ͬʱʹ�ã�ԭ���ǣ������ӷ���ʽ��ʾ��____________��
�������ܱձ�������ԭ��____________________________________________��
��7����ҵ�����������ƺ�ϡ����Ϊԭ���Ʊ� ClO2 ��д����Ӧ�Ļ�ѧ����ʽ____________��Cl2��ClO2����ǿ������,��ɱ��ˮ�еIJ�������������ClO2������������Cl2��_______����
��8����(CN)2������(SCN)2�Ļ�ѧ���ʺ�±��(X2)�����ƣ���ѧ�ϳ�Ϊ��±�أ��磺[(SCN)2��H2O = HSCN��HSCNO]�����������ӵĻ�ԭ��ǿ��Ϊ Cl-<Br-<CN-<SCN-<I-����д����KBr��KSCN�Ļ����Һ�м���(CN)2����Ӧ�Ļ�ѧ����ʽΪ_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��һ����ʵ��Ϊ������ѧ�ƣ��Իش��������⣺
I����������Ϊ36.5%��Ũ����(�ܶ�Ϊ1.16 g��cm��3)���Ƴ�1 mol��L��1��ϡ���ᡣ��ʵ���ҽ���Ҫ��������220 mL���Իش��������⣺
��1������ϡ����ʱ��Ӧѡ������Ϊ________mL������ƿ��
��2����������Ҫ________mLŨ���ᣬ����ȡʱ��ѡ��������Ͳ�е�________��
A��5 mL B��10 mL C��25 mL D��50 mL
��3������ȡŨ������������в�����
�ٵ�ϡ�͵�������¶�������һ�º��ز�����ע��250 mL����ƿ�С�
��������ƿ��С�ļ�����ˮ��Һ��������ƿ�̶���1��2 cmʱ�����ý�ͷ�ιܼ�����ˮ��ʹ��Һ��Һ����ƿ���Ŀ̶ȱ������С�
����ʢ������ձ���ע������ˮ�����ò�����������ʹ���Ͼ��ȡ�
��������ˮϴ���ձ��Ͳ�����2��3�Σ�����ϴ��Һȫ��ע������ƿ��
���������У���ȷ��˳����(�����)____________��
��4�����������ƹ����У��øո�ϴ�ӽྻ����Ͳ����ȡŨ���ᣬ�����Ƶ�ϡ����Ũ��________(����ƫ��������ƫ����������Ӱ����)����δ������ˮϴ���ձ��ڱڻ�δ��ϴ��Һע������ƿ�������Ƶ�ϡ����Ũ����________(����ƫ��������ƫ����������Ӱ����)��
IIʵ����������װ�ý���ʵ��
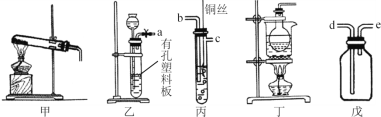
��5��װ�ü���������ȡ���ռ��������������з�����Ӧ�Ļ�ѧ����ʽΪ�� ______________________�� ��װ���ռ�����Ӧ���ռ�װ�õ�_____(����ĸ���)���ܽ�����
��6��ѡ��װ���ҡ��������Ʊ����ռ�һ���������壬�������ϰ���������ʯ��ʯ������ ʢ��ϡNaOH��Һ��������ȷ������˳��Ϊ________________________ (�ýӿ���ĸ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�����ӷ���ʽ��д��ȷ����( )
A. ̼��������ᷴӦ��CO32��+2H+=H2O+CO2��
B. ����������ˮ�Ʊ������Cl2��H2O =2H����Cl����ClO��
C. ����������Һ��ϡH2SO4 ��Ӧ��Ba2++SO42��=BaSO4��
D. �Ȼ�ͭ��Һ�����۷�Ӧ��Cu2++Fe=Fe2++Cu
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������FeC2O4��2H2O���������Ʊ�������﮵���������ϡ�ʵ�����Ʊ������������ⶨ����ɵ�ʵ���������£�

��1�� ���ܽ���ʱ���ȵ�Ŀ����_____________________����������ʱ����Һ��в��ڲ��Ͻ����¼���H2C2O4��Һ�������Ͻ���������ʹ��Ӧ���ֽӴ��⣬��һĿ����_____________________��
��2�� �����ˡ�ϴ����ʱ����˵��������ϴ�Ӹɾ���������_____________________��
��3�������²�����Բⶨ��Ʒ�в����������������
�ٳ�ȡ0.1600g������������ƿ�У�����25mL2mol��L��1��H2SO4��Һ��������40~50����ʹ��Ʒ�ܽ⡣
����0.02000 mol��L��1KMnO4��Һ�ζ����յ㣬����KMnO4��Һ23.80mL��
[5C2O![]() +2MnO
+2MnO![]() +16H+=10CO2��+2Mn2++8H2O��5Fe2++MnO
+16H+=10CO2��+2Mn2++8H2O��5Fe2++MnO![]() +8H+=5Fe3++Mn2++4H2O]
+8H+=5Fe3++Mn2++4H2O]
���ڢڵζ������Һ�м�������Zn����2Fe3++Zn = 2Fe2++Zn2+����5mL2mol��L��1 ��H2SO4��Һ�����Լ10min��
�ܽ���Һ��������һ����ƿ�У���10mL1mol��L��1��H2SO4��Һϴ����ƿ����ȫ��Fe2+ת������ƿ�У�����0.02000 mol��L��1KMnO4��Һ�ζ����յ㣬����KMnO4��Һ���8.00 mL��
��I����õIJ�Ʒ��n(Fe![]() ) ��n(C2O
) ��n(C2O![]() ) _________1��1������>�� ����������<������
) _________1��1������>�� ����������<������
��II��������Ʒ��C2O![]() ����������(д���������)��______________
����������(��������)��______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������A2 + 3B2![]() 2C+D�ķ�Ӧ��˵�����»�ѧ��Ӧ���ʵı�ʾ�У���Ӧ���������ǣ� ��
2C+D�ķ�Ӧ��˵�����»�ѧ��Ӧ���ʵı�ʾ�У���Ӧ���������ǣ� ��
A��v(B2) =0.8mol(Ls)-1 B��v(A2) =0.4 mol(Ls)-1
C��v(C) =0.6 mol(Ls)-1 D��v(D) =0.1 mol(Ls)-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������������������ȷ����
A. 32 g O2��O3�Ļ����������ԭ����ΪNA
B. 5NH4NO3![]() 2HNO3+4N2��+9H2O��Ӧ�У�����28g N2ʱ��ת�Ƶĵ�����ĿΪ3.75NA
2HNO3+4N2��+9H2O��Ӧ�У�����28g N2ʱ��ת�Ƶĵ�����ĿΪ3.75NA
C. �����£�0.2mol Fe������ˮ������Ӧ�����ɵ�H2������ĿΪ0.3NA
D. ��25�桢101kPa�£�2 mol�����������Ļ����������ԼΪ44.8 L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����400mLNH4HCO3��Na2CO3�Ļ����Һ�ֳ����ȷݣ�ȡһ�ݼ��뺬a mol�������Ƶ���Һǡ�÷�Ӧ��ȫ����һ�ݼ��뺬b mol HCl������ǡ�÷�Ӧ��ȫ����û����Һ��c(Na+)Ϊ
A. (b/10-a/20)mol/L B. (2b-a)mol/L C. (5b-5a/2)mol/L D. (10b-5a)mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС������ʵ����̽�����������ʼ�ģ�ҵ��ȡƯ�ۣ����������װ�ý���ʵ�顣
�밴Ҫ��ش��������⣺
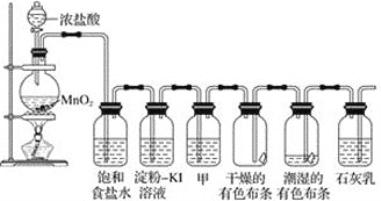
(1)����ʳ��ˮ��������_______________________________��
(2)����-KI��Һ�й۲쵽��������_____________����Ӧ�����ӷ���ʽΪ_______________��
(3)���������ɫ��������ɫ��ʪ�����ɫ������ɫ���ɸ�����ɵó��Ľ�����___________________����ʢ�ŵ��Լ���____________��
(4)Cl2��ʯ���鷴Ӧ��ȡƯ�۵����ӷ���ʽΪ________________________��
(5)����ȤС����17.4 g ����������������Ũ�����Ʊ���������Ӧ�����ӷ���Ϊ____________�������������Ƶñ�״���������������_______L��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com