
| ʵ�鲽�裨��Ҫ��д����������̣� | Ԥ������ͽ��� |
| | ����Һ����������2������ ����Һ������������2�������� |
| ���� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

A����0.1mol��L��1 Na2CO3��Һ�У������η�̪��dz��ɫ���Ⱥ��ɫ���˵������ˮ�ⷴӦ�����ȷ�Ӧ | B���ò�˿պȡ����ij��Һ������ɫ��Ӧ������ɫ�ܲ����۲쵽�������ɫ������Һһ���Ǽ�����Һ |
| C����ijFeCl2��Һ�м���KSCN��Һ���۲쵽��Һ��Ѫ��ɫ��˵������Һ��FeCl2��ȫ�������� | |
| D����ij��Һ�м���ϡ���ᣬ����������ͨ�����ʯ��ˮ��ʯ��ˮ����ǣ�����Һһ������CO32�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

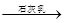 Mg��OH��2
Mg��OH��2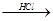 MgCl2��Һ��MgCl2��6H2O��MgCl2
MgCl2��Һ��MgCl2��6H2O��MgCl2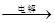 Mg
Mg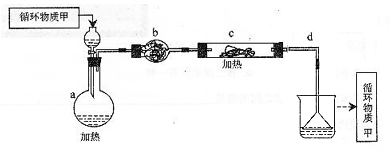
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
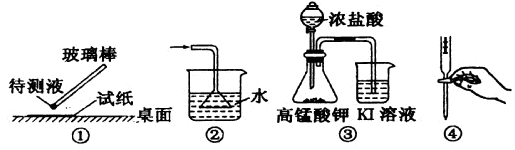
| A������pH��ֽ��ij��Һ������� | B�������հ����ư�ˮ |
| C����̽�������ԣ�KMnO4>Cl2>I2 | D�����к͵ζ�ʵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ԥ�����ʵ����� | B���۲����ʵ����״̬ |
| C������ʵ��۲� | D��������صĽ��ͺͽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
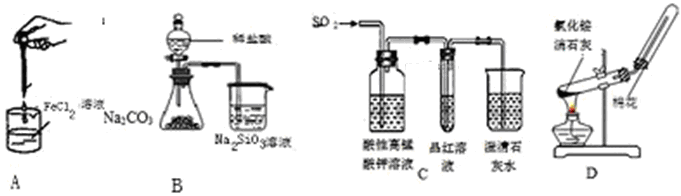
| A���Ʊ����������� | B����֤�ǽ�����Cl >C >Si |
| C����������������Ƿ���ж�����̼ | D��ʵ������ȡ���ռ����� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com