
����Ŀ��ClO2��Cl2�������������������ˮ�������߱��ʵȷ���Ӧ�ù㷺��ij��ȤС��ͨ��ͼ1װ�ã��г�װ���ԣ������Ʊ������ա��ͷź�Ӧ�ý������о�������֪��װ��C������������Cl2��
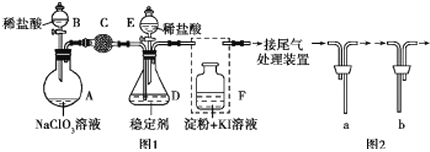
(1)����B��������________����װF�е���ʱ��Ӧѡ��ͼ2�е�_________________��
(2)��B�Ļ�����A�з�����Ӧ��2NaClO3+4HCl�T2ClO2��+Cl2��+2NaCl+2H2O��ΪʹClO2��D�б��ȶ���������գ��μ�ϡ������ٶ���_______________����������������������
(3)�ر�B�Ļ�����ClO2��D�б��ȶ�����ȫ��������NaClO2��NaClO2��Cl�Ļ��ϼ�Ϊ______��
(4)��֪������������NaClO2�ɷ�����Ӧ����NaCl���ͷų�ClO2���÷�Ӧ�����ӷ���ʽΪ__________��
(5)��ҵ�Ͽ���KClO3��Na2SO3��H2SO4�������Ƶ�ClO2���÷�Ӧ���ӷ���ʽΪ___________________������֪��������������SO32����ClO3������ΪSO42����
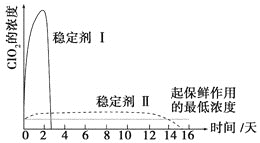
(6)������ClO2������ȶ��������ȶ�������������ͷ�ClO2��Ũ����ʱ��ı仯��ͼ3��ʾ������������ˮ�����ʣ�����ΪЧ���Ϻõ��ȶ�����__________��ԭ����________________��
���𰸡���Һ©�� b �� ��3 5ClO2����4H+��Cl����4ClO2��2H2O 2H+��2ClO3����SO32����SO42����2ClO2��H2O �ȶ����� ���ȶ��������������ܽϳ�ʱ�䱣��
��������
װ��A��ʢ��NaClO3��Һ��װ��Bʢ��ϡ���ᣬ���߷�����Ӧ��2NaClO3+4HCl=2ClO2��+Cl2��+2NaCl+2H2O��װ��C������������Cl2����ȥ������ClO2��װ��D�б��ȶ�����ȫ��������NaClO2��������������NaClO2�ɷ�����Ӧ4H++5ClO2-=Cl-+4ClO2��+2H2O����NaCl���ͷų�ClO2��װ��F������������������Ƿ���ȫ��װ��C���գ�
(1) ���������ṹ������ȷ������B�����ƣ�Fװ��Ӧ��Cl2��KI��Ӧ������Ҫ����β������װ�ã�����Ӧ���ܽ������̹ܳ�����
(2) ΪʹClO2��D�б��ȶ���������գ�����ClO2������Ҫ����
(3) NaClO2��NaΪ+1�ۣ�OΪ-2�ۣ������������ϼ۴�����Ϊ0���ж�Cl�Ļ��ϼۣ�
(4) ������������NaClO2�ɷ�����Ӧ����NaCl���ͷų�ClO2������Ԫ���غ��֪Ӧ����ˮ���ɣ�
(5) KClO3��H2SO4��������Na2SO3��Ӧ��SO32-��������SO42-���ɵ��ӡ�����غ�д��������Ӧ�����ӷ�Ӧ����ʽ��
(6) ��ͼ��֪���ȶ�������Ի����ͷ�ClO2���ܽϳ�ʱ��ά�ֱ��������Ũ�ȡ�
(1)����B�������������Ҵ��в���������Ϊ��Һ©����Fװ��Ӧ��Cl2��KI��Ӧ������Ҫ����β������װ�ã�����Ӧ���ܽ������̹ܳ�������ѡb��
(2) ΪʹClO2��D�б��ȶ���������գ�����ClO2������Ҫ�����ʵμ�ϡ������ٶ�Ҫ����
(3) NaClO2��NaΪ+1�ۣ�OΪ-2�ۣ������������ϼ۴�����Ϊ0����Cl�Ļ��ϼ�Ϊ+3��
(4) ������������NaClO2�ɷ�����Ӧ����NaCl���ͷų�ClO2������Ԫ���غ��֪Ӧ����ˮ���ɣ��÷�Ӧ�����ӷ���ʽΪ��4H++5ClO2-=Cl-+4ClO2��+2H2O��
(5) KClO3��H2SO4��������Na2SO3��Ӧ�Ƶ�ClO2����֪SO32-��������SO42-���ɵ��ӡ�����غ��֪�����ӷ�ӦΪ2ClO3-+SO32-+2H+�T2ClO2+SO42-+H2O��
(6) ��ͼ��֪���ȶ�������Ի����ͷ�ClO2���ܽϳ�ʱ��ά�ֱ��������Ũ�ȣ������ȶ�����á�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�Ͽ��ü����������9��1������Ȼ�ϣ���200����10MPa�������£�ͨ��ͭ�ƹܵ���Ӧ�Ƶü״���2CH4+O2=2CH3COH��
��1����֪һ�������£�CH4��CH3COHȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
CH4(g)+SO2(g)=CO2(g)+2H2(g) ��H=-802kJ/lmol
CH3OH(g)+l.5O2(g)=CO2(g)+2H2O(g) ��H=-677kJ/mol
��2CH4(g)+O2(g)=2CH3OH(g) ��H=__________
��2���������������ȼ�ϵ�����ڵ�����ȼ�ϵ�أ���һ�����и�����ֱ�ӽ�������ȼ�Ϻ��������еĻ�ѧ�ܸ�Ч�������Ѻõ�ת���ɵ��ܵ�ȫ��̬��ѧ����װ�á��乤��ԭ������ͼ��ʾ��a�ǵ�ص�____��������������������) ��b���ĵ缫��ӦʽΪ__________��

��3����ҵ�Ϻϳɼ״�����һ�ַ���Ϊ��
CO(g)+2H2(g) ![]() CH3OH(g) ��H��-90kJ/mol
CH3OH(g) ��H��-90kJ/mol
T��ʱ����2mol CO ��4molH2����1L ���ܱ������У����H2�����ʵ�����ʱ��仯����ͼʵ����ʾ��
�� �������жϷ�Ӧ�Ѵﻯѧƽ��״̬����____________��
A���������ѹǿ���ٸı�
B. H2��CH3OH������Ȳ��ٸı�
C��������ܶȲ��ٸı�
D����λʱ��������1mol CO,ͬʱ����1mol CH3OH
�� ������T�� ʱ��Ӧ��ƽ�ⳣ��K=_________________��
�� ���ı�ijһʵ�������ٽ�������ʵ����H2�����ʵ�����ʱ��仯��ͼ��������ʾ����������Ӧ��ʵ�������ı��ǣ�________����������Ӧ��ʵ�������ı���_________0��

�� a��b��c�����淴Ӧ�����ɴ�С���е�˳����__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ĩ״����A���ɵ����ʵ�����MgO��Fe2O3��ɵĻ�����������ʵ�飺
��ȡ����A�������ȷ�Ӧ���������е���B���ɣ�
����ȡ20 g Aȫ������0.15 L 6.0 mol��L��1�����У�����ҺC��
�������еõ��ĵ���B����ҺC��Ӧ���ų�1.12 L(��״��)���壬ͬʱ������ҺD���������й�������B��
����KSCN��Һ����ʱ����ҺD����ɫ��
����գ�
(1)�����������ȷ�Ӧ��ʵ������ǣ�____________�������е���B��________��
(2)�����������ĸ���Ӧ�Ļ�ѧ����ʽΪ____________________��________________��
(3)�����������ĸ���Ӧ�����ӷ���ʽΪ___________________��_____________________��
(4)����ҺD���������Ϊ0.15 L�������Һ��c(Mg2��)Ϊ________��c(Fe2��)Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
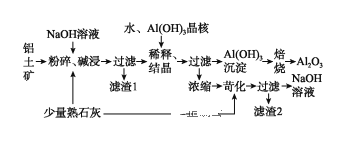
����Ŀ��ij����������Ҫ����Al2O3��Al(OH)3������Fe2O3�����ʡ����ðݶ���������������������ͼ��ʾ:
��ش���������:
(1)��������������ʱӦ�ڽϸ��¶��½��У���Ŀ����____________________������1����Ҫ�ɷ�Ϊ________________��
(2)Al2O3��NaOH��Һ��Ӧ�����ӷ���ʽΪ____________________________________��
(3)��Al(OH)3���˵�Ŀ���Ǵٽ�Al(OH)3��������������ϡ�͡��ᾧ�����գ�Ҳ����ͨ��������_________����ķ��������档����֪��ͨ�������������֮һ�����������ͷۣ���
(4)���չ����з����Ļ�ѧ����ʽΪ_________________________________��
(5)Ũ�����õ�NaOH��Һ���������˿����е�CO2���������ʣ������ʿ�ͨ������������������ʯ�ҷ�Ӧ����Ӧ��ȥ��д��������Ӧ�Ļ�ѧ����ʽ��_________________________________________________��
(6)������������ʵ��____________________���ѧʽ����ѭ�����á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
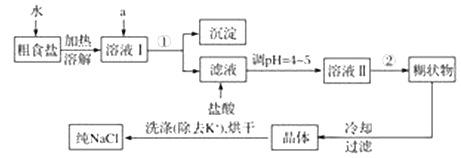
����Ŀ��ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ���ʳ���г���������K+��Ca2+��Mg2+��Fe3+��SO42�����������ӣ�ʵ�����ᴿNaCl����������:
�ṩ���Լ�������Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ��75%�Ҵ���
(1)�ڹ��˹���������Ҫ�IJ���������________________________________________��
(2)����ȥ��Һ���е�Ca2+��Mg2+��Fe3+��SO42����ѡ��a���������Լ������μ�˳������Ϊ_______��ֻ�ѧʽ����
(3)���ڳ������Լ�������������ܽ����ʳ�����������˵���Һ���Ƿ���Fe3+�ķ���__________���������ó�����Ҫϴ�ӣ�ϴ�ӳ����ķ���__________________��
(4)���ᴿ��NaCl���Ƴ�480ml 0.2molL��1��NaCl��Һ����������ƽ��ȡ___________ g����NaCl���壬������ˮ��_______________���ܽ⣬��ȫ�ܽ��ȫ��ת����________________�У�������ˮ��______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ȡ�طʺ�ұ���������幤����������ͼ��ʾ��
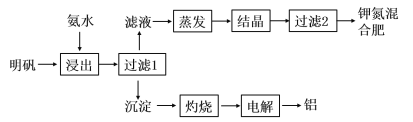
��1������������Ӧ�����ӷ���ʽΪ__________________________________��������������ϡ��ˮŨ��Ϊ6mol/L��������100mL�ð�ˮ��12mol/L��Ũ��ˮ�����Ϊ______mL������ȡ��Ũ��ˮʱ���ӿ̶��ߣ��ᵼ�����Ƶ�ϡ��ˮŨ��________���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��2�����顰����1�����ó����Ƿ�ϴ����ʵ�鷽����_______________________________��
��3����д���������������������ȡ�������Ļ�ѧ����ʽ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ��ɫ��Һ�����п��ܺ���Fe3+��Al3+��Fe2+��Mg2+��Cu2+��NH4����K+��CO32����SO42�������ӵļ��֣�Ϊ������ɷ֣�ȡ����Һ�ֱ�������ĸ�ʵ�飬��������й�������ͼ��ʾ��

���������ͼ�ƶ���
��1��ԭ��Һ��һ�����ڵ���������_______________����___(����������������������)�ԡ�
��2��ʵ����в�����ɫ��ζ�����������Ļ�ѧ����ʽΪ__________________________________��
��3��д��ʵ�����A���Ӧ�����Ļ�ѧʽ��__________��
��4��д��ʵ���������A��B��������������Ӧ�����ӷ���ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һδ֪����ɫ��Һ��ֻ���ܺ������������е�������(������ˮ���������H����OH��)��H����NH![]() ��K����Mg2����Cu2����Al3����NO
��K����Mg2����Cu2����Al3����NO![]() ��CO
��CO![]() ��SO
��SO![]() ����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼ�����AgNO3��Һ���а�ɫ�����������ڵڶ��ݼ�����BaCl2��Һ���а�ɫ������������ϴ�ӡ������������Ϊ6.99 g���۵�������εμ�NaOH��Һ����ó�����NaOH��Һ�������ϵ��ͼ����������ʵ�飬�����Ʋⲻ��ȷ����
����ȡ����100 mL��Һ��������ʵ�飺�ٵ�һ�ݼ�����AgNO3��Һ���а�ɫ�����������ڵڶ��ݼ�����BaCl2��Һ���а�ɫ������������ϴ�ӡ������������Ϊ6.99 g���۵�������εμ�NaOH��Һ����ó�����NaOH��Һ�������ϵ��ͼ����������ʵ�飬�����Ʋⲻ��ȷ����

A. ԭ��Һһ��������H����Cu2����CO![]()
B. ����ȷ��ԭ��Һ�Ƿ���K����NO![]()
C. ԭ��Һȷ����Mg2����Al3����NH![]() ����n(Mg2��) �U n(Al3��) �U n(NH
����n(Mg2��) �U n(Al3��) �U n(NH![]() )��1�U1�U2
)��1�U1�U2
D. ʵ�����ӵ�NaOH��Ũ��Ϊ2 mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ҩ������������ǿ��������ܰ�ϸ����ֳ�����ã���ͨ�����·�Ӧ�Ƶã�
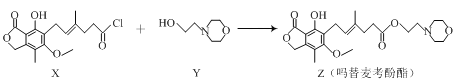 +HCl
+HCl
����������ȷ����
A.����ˮ�ɼ�����X��ZB.������Y�ķ���ʽΪC6H12NO2
C.������Z�к�������̼ԭ��D.1mol������Z������3molNaOH��Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com