
| A�� | 2N2H4��g��+2NO2��g���T3N2��g��+4H2O��l����H=-1135.7 kJ•mol-1 | |
| B�� | 2N2H4��g��+2NO2��g���T3N2��g��+4H2O��g����H=-1000.3 kJ•mol-1 | |
| C�� | N2H4��g��+NO2��g���T$\frac{3}{2}$N2��g��+2H2O��l����H=-1135.7 kJ•mol-1 | |
| D�� | 2N2H4��g��+2NO2��g���T3N2��g��+4H2O��g����H=-1135.7 kJ•mol |
���� ���ݸ�˹���ɣ�����֪�Ȼ�ѧ����ʽ���Ժ��ʵ�ϵ�����мӼ�����Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ��ϵ��������Ӧ�����㣮
��� �⣺��֪����N2��g��+2O2��g��=2NO2��g������H=+67.7kJ/mol
��N2H4��g��+O2��g��=N2��g��+2H2O��g������H=-534kJ/mol
���ݸ�˹���ɣ��ڡ�2-�ٵ�2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g�����ʡ�H=2����-534kJ/��-67.7kJ/mol=-1135.7 kJ/mol��
�º�NO2����N2��ˮ�������Ȼ�ѧ����ʽΪ��2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g������H=-1135.7 kJ/mol��
A��ˮ��״̬ӦΪ��̬������Һ̬����A����
B����Ӧ��Ϊ��H=-1135.7 kJ/mol�����ǡ�H=-1000.3 kJ/mol����B����
C��1molN2H4��g����Ӧ��ˮ��״̬ӦΪ��̬������Һ̬����Ӧ��Ϊ��H=-567.86 kJ/mol����C����
D��������������֪���º�NO2����N2��ˮ�������Ȼ�ѧ����ʽΪ��2N2H4��g��+2NO2��g��=3N2��g��+4H2O��g������H=-1135.7 kJ/mol����D��ȷ��
��ѡD��
���� ���⿼���Ȼ�ѧ����ʽ����д���ѶȲ���ע����д�������˹���ɵ����ã�


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ڢ� | C�� | �٢� | D�� | �ܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH=CH2+Br2$\stackrel{CCl_{4}}{��}$CH3CHBrCH2Br | |
| B�� | CH3CH2OH $��_{170��}^{ŨH_{2}SO_{4}}$ CH2=CH2��+H2O | |
| C�� | CH3COOH+CH3CH2OH $��_{��}^{ŨH_{2}SO_{4}}$ CH3COOCH2CH3+H2O | |
| D�� | 2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol Na������ˮ��Ӧ��ת�Ƶ�����ΪNA | |
| B�� | ͬ��ͬѹ�£���ͬ��������ʣ������ʵ�������� | |
| C�� | 5.3g Na2CO3���庬�е�������Ϊ1.5NA | |
| D�� | ��״���£�22.4L H2O������ԭ����Ϊ3 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
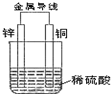
| A�� | ͭƬ�������ݲ��� | B�� | ͭƬ�������� | ||
| C�� | ���Ӵ�пƬ����������ͭ | D�� | ��װ�ðѻ�ѧ��ת��Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ̼�������仯�����ڹ�ũҵ����������������Ҫ���ã���ش��������⣺
̼�������仯�����ڹ�ũҵ����������������Ҫ���ã���ش��������⣺�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com