
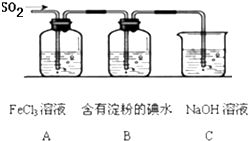
ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ������ͼ��ʾ��
��1��SO2���廹ԭFe3+�IJ����� �������ӷ��ţ����μӷ�Ӧ��SO2��Fe3+�����ʵ���֮���� ��
��2������ʵ�鷽����������ʵ������ȡ����SO2���� ������ţ���
A��Na2SO3��Һ��HNO3 B��Na2SO3������Ũ����
C���������ڴ�����ȼ�� D���������ڸ�������O2��Ӧ
��3��װ��C�������� ��
��4����Ҫ��A��������Һ��ȡ���壬������е�ʵ��������裺��������ȴ�ᾧ�����ˡ���Ȼ�������һϵ�в�����û���õ��������� ������ţ���
A�������� B��ʯ���� C��©�� D���ձ� E�������� F������
��5��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ������ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м���KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
�����ۣ�����������Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������ ��ԭ���� ��
��6���ܱ���I���Ļ�ԭ������SO2�������� ��
��16�֣���1��SO42-��Fe3+�� 1�U2 ��2��B
��3������SO2β������ֹ��Ⱦ���� ��4��BF
��5�������٣���ΪA����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ
��6��B����ɫ��Һ��ɫ ��ÿ��2�֣�
���������������1��SO2���廹ԭFe3+�IJ�����SO42-��Fe3+����Ӧ�ķ���ʽ��SO2��2Fe3����2H2O=2Fe2����SO42����4H�����μӷ�Ӧ��SO2��Fe3+�����ʵ���֮��1�U2��
��2��������������ԣ����������Ʒ�Ӧ�ò���SO2��Na2SO3������Ũ���ᷴӦ����SO2��B��ȷ��ѡ��CD����Ȼ��������SO2�����õ������岻�Ǵ�������ѷ��룬��ѡB��
��3��SO2�Ǵ�����Ⱦ�����Cװ�õ�����������SO2β������ֹ��Ⱦ������
��4����Һ����Ũ��Ӧ���������������������Ҳ���Ҫ��ʯ��������˴�ѡBF��
��5������A����Һ�к���SO2��SO2Ҳ��ʹKMnO4��Һ��ɫ�����Է������Dz������ġ�
��6��SO2��ʹ���ʵԭ����B����ɫ��Һ��ɫ�Ϳ���˵��I���Ļ�ԭ������SO2�ġ�
���㣺����������ԭ��Ӧ���й��жϺͼ��㡢������Ʊ���β�������Լ�������ѡ���SO2����̽��ʵ����й��ж�
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ��ʵ�������ĺͽ��ⷽ����ָ����ѵ��������������ѧ���淶���Ͻ���ʵ����ƺ���������������ѧ����ѧ�����������������ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ��
ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ���ݶ�ݸ��У������ѧ�ڵ�һ�����������ۣ���ѧ ���ͣ�ʵ����
��16�֣�ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ������ͼ��ʾ��
FeCl3��Һ ���е��۵ĵ�ˮ NaOH��Һ
A B C
��1��SO2���廹ԭFe3+�IJ����� �������ӷ��ţ����μӷ�Ӧ��SO2��Fe3+�����ʵ���֮���� ��
��2������ʵ�鷽����������ʵ������ȡ����SO2���� ������ţ���
A��Na2SO3��Һ��HNO3 B��Na2SO3������Ũ����
C���������ڴ�����ȼ�� D��ͭ����ŨH2SO4
��3��װ��C�������� ��
��4����Ҫ��A��������Һ��ȡ���壬������е�ʵ��������裺��������ȴ�ᾧ�����ˡ���Ȼ�������һϵ�в�����û���õ��������� ������ţ���
A�������� B��ʯ���� C��©�� D���ձ� E�������� F������
��5��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ������ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м���KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
�����ۣ�����������Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������ ��ԭ���� ��
��6���ܱ���I���Ļ�ԭ������SO2�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��㶫ʡ��ͷ�и�����ѧ����ĩ�������ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ������ͼ��ʾ��

FeCl3��Һ ���е��۵ĵ�ˮ NaOH��Һ
A B C
��1���������Ȼ�����Һʱ�����Ȱ��Ȼ��������ܽ��� �У��ټ�ˮϡ�ͣ�����������Ŀ���� ��
��2��װ��C�������� ��
��3����Ҫ��A��������Һ��ȡ���壬������е�ʵ��������裺��������ȴ�ᾧ�� ����Ȼ�������һϵ�в�����û���õ��������� ������ţ���
A��������
B��ʯ����
C��©��
D���ձ�
E��������
F�� ����
��4��������������С��ͬѧ��ΪSO2 �� FeCl3��Һ����������ԭ��Ӧ��
��д��SO2��FeCl3��Һ��Ӧ�����ӷ���ʽ ��
�������ʵ�鷽��������Fe2+���� ��
�۸�С��ͬѧ��C�ձ���Ӧ�����Һ�м��������ữ��BaCl2��Һ�������ְ�ɫ����������֤����Ӧ������SO42���������� ��������������������������� ��
��5���ܱ���I���Ļ�ԭ������SO2�������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com