
(1)һ�������£�������ӦCO(g)��H2O(g)  CO2(g)��H2(g)����2 L�ܱ�������ͨ��2 mol CO(g)��1 mol H2O(g)��2 min ��Ӧ�ﵽƽ��ʱ�����CO�����ʵ���Ϊ1.6 mol����H2O(g)��ʾ�÷�Ӧ����Ϊ____________�����¶��£��÷�Ӧ��ƽ�ⳣ��Ϊ________�������������ټ���2 mol CO(g)�������´ﵽƽ��ʱ��CO��ת����________20%(�����������������)��
CO2(g)��H2(g)����2 L�ܱ�������ͨ��2 mol CO(g)��1 mol H2O(g)��2 min ��Ӧ�ﵽƽ��ʱ�����CO�����ʵ���Ϊ1.6 mol����H2O(g)��ʾ�÷�Ӧ����Ϊ____________�����¶��£��÷�Ӧ��ƽ�ⳣ��Ϊ________�������������ټ���2 mol CO(g)�������´ﵽƽ��ʱ��CO��ת����________20%(�����������������)��
(2)��һ�ܱ������з�����Ӧ2NO2 2NO��O2����H��0����Ӧ������NO2��Ũ����ʱ��仯���������ͼ��ʾ��
2NO��O2����H��0����Ӧ������NO2��Ũ����ʱ��仯���������ͼ��ʾ��
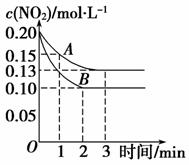
������A��B�ֱ��ʾ���Ǹ÷�Ӧ��ij��ͬ�����µķ�Ӧ�����������B������������________(�����ѹǿ��������Сѹǿ�����������¶ȡ����������¶ȡ���ʹ �ô�����)��
����)��
(3)һ���¶��£����ܱ�������N2O5�ɷ������з�Ӧ��
��2N2O5(g)  4NO2(g)��O2(g)
4NO2(g)��O2(g)
��2NO2(g)  2NO(g)��O2(g)
2NO(g)��O2(g)
����ƽ��ʱ��c(NO2)��0.6 mol��L��1��c(O2)��1.3 mol��L��1����Ӧ����NO2��ת����Ϊ________��
������(1)v(H2O)��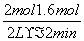 ��0.1 mol��L��1��min��1��K��
��0.1 mol��L��1��min��1��K��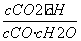 ��
��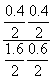 ��0.166 7���ټ���CO��������ת����Ҫ��С��
��0.166 7���ټ���CO��������ת����Ҫ��С��
(3)���£�ƽ�����ƣ�c(NO2)��С��
2N2O5(g)  4NO2(g)��O2(g)
4NO2(g)��O2(g)
a 0 0
a��2x 4x x
2NO2(g)  2NO(g)��O2(g)
2NO(g)��O2(g)
4x 0 0
4x��2y 2y y
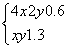 ��
��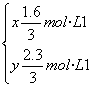
���ԣ�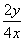 ��100%��
��100%��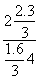 ��100%��71.88%��
��100%��71.88%��
�𰸡�(1)0.1 mol��L��1��min��1��0.166 7����
(2)�����¶ȡ�(3)71.88%


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ�к��ж��ֳɷ֣�������ж���
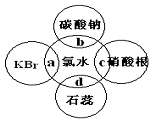 ���ʣ�������ˮ�ֱ�����ͼ�������ʷ����ķ�Ӧ
���ʣ�������ˮ�ֱ�����ͼ�������ʷ����ķ�Ӧ
��գ�a��b��c��d�غϲ��ִ������ʼ䷴Ӧ������ˮ
��������
��֤����ˮ����Ư���Ե���__________(�a������b������c����d��)��
��1��C�����е�������_______________________��b�����е����ӷ���ʽΪ
_____________________________________����ʾ������H2CO3 > HClO > HCO3-����
��2��a�����еĻ�ѧ����ʽΪ________________________________________��
��4��ij�¶��£���Cl2ͨ��NaOH��Һ�У���Ӧ�õ�NaCl��NaClO��NaClO3�Ļ��Һ�����ⶨClO����ClO3����Ũ��֮��Ϊ1��3����Cl2��NaOH��Һ��Ӧʱ����ԭ����Ԫ���뱻��������Ԫ�ص����ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NA��ʾ�����ӵ�����������˵����ȷ���ǣ� ��
A��1 mol Na������O2��Ӧ����Na2O��Na2O2��ʧȥNA������
B��1 mol Al�ֱ�������������ŨHNO3��ϡHNO3�У���Ӧ��ת�Ƶĵ��Ӿ�Ϊ3NA
C����5.6 g���ֱ������������ᡢ������Ӧ������ת��������Ϊ0.3NA
D��24 gþ�������������NaOH��Һ��Ӧת�Ƶĵ�����Ŀ��Ϊ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ����Ȼ�ѧ����ʽΪN2(g)��3H2(g)  2NH3(g
2NH3(g )����H����92.4 kJ��mol��1���ֽ�1 mol N2(g)��3 mol H2(g)����һ�ݻ�Ϊ2 L���ܱ������У���500 ���½��з�Ӧ��10 minʱ�ﵽƽ�⣬NH3���������Ϊ��������˵������ȷ����
)����H����92.4 kJ��mol��1���ֽ�1 mol N2(g)��3 mol H2(g)����һ�ݻ�Ϊ2 L���ܱ������У���500 ���½��з�Ӧ��10 minʱ�ﵽƽ�⣬NH3���������Ϊ��������˵������ȷ����

A�����ﵽƽ��ʱ�������ϵ�ų�9.24 kJ��������H2��Ӧ���ʱ仯������ͼ����ʾ
B����Ӧ�����У��������ƽ����Է�������ΪM����������ܶ�Ϊd���������ѹǿΪp�����߹�ϵ��ͼ��
C����ͼ����ʾ��������͢�ﵽƽ��ʱ����Ҫ��ʱ����ܲ�ͬ
D������ʼ��������Ϊ1 mol N2��3 mol H2���ڲ�ͬ�����´ﵽƽ��ʱ��NH3����������仯��ͼ����ʾ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ӦN2O4(g)2NO2(g)����H����57 kJ��mol��1�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�ı仯������ͼ��ʾ������˵����ȷ����

A��a��c����ķ�Ӧ���ʣ�a��c
B��a��c�����������ɫ��a�cdz
C����״̬b��״̬a�������ü��ȵķ���
D��a��c���������ƽ����Է���������a��c
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����л�����з��������һ�ֳɷ֣�����ȡ���뷽����ȷ����(����)
A�����ڵ��ھƾ��е��ܽ�ȴ����Կ��þƾ��ѵ�ˮ�еĵ���ȡ����
B��ˮ�ķе���100 �棬�ƾ��ķе���78.5 �棬���Կ��ü�������ʹ��ˮ�ƾ���Ϊ��ˮ�ƾ�
C���Ȼ��Ƶ��ܽ�����¶��½�����С����������ȴ�����ȵĺ��������Ȼ��ص��Ȼ���Ũ��Һ�еõ��������Ȼ��ƾ���
D�����ڽ�����ֱ�������Ӵ����Ե����л��еĵ⻯�ؿ�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��Ԥ����ȱ�������йز��Ź涨ʳ���еĵ⺬��(��I��)Ӧ��20��50 mg/kg���Ʊ�KIO3�ķ������£�
����1��6I2��11KClO3��3H2O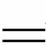 6KH(IO3)2��5KCl��3Cl2��
6KH(IO3)2��5KCl��3Cl2��
KH(IO3)2��KOH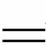 2KIO3��H2O
2KIO3��H2O
����2�����������£�KI��3H2O KIO3��3H2��
KIO3��3H2��
����3��I2 HIO3
HIO3 KIO3
KIO3
(1)�뷽��3��ȷ���1�IJ�����________________________________________________________________________��
����2�IJ�����________________________________________________________________________��
(2)����2ѡ�õĵ缫�Ƕ��Ե缫������������Ӧʽ��________________________________________________________________________��
(3)����3��Ӧ�¶ȿ�����70 �����ң������ø����¶ȵ���Ҫԭ����________________________________________________________________________��
(4)�Ʊ�����KIO3��ʵ�鲽���У�����轫���þ������ʹ��____________________ϴ��2��3�Σ������ò�Ʒ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵���������(����)
A��ʯ���к���C5��C 11������������ͨ��ʯ�͵ķ���õ�����
11������������ͨ��ʯ�͵ķ���õ�����
B����C18�������������;������ѽ���Եõ�����
C��ú�����л����������ɵĸ��ӵĻ����
D��ú�к��б��ͼױ����������ȸ�������ķ��������Ƿ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���� �� ��
A��ʯ��Һ��������Ҫ�ɷ�Ϊ���� B��ú�ĸ���ɷ����ú�к��е�ú����
C��ʯ�͵ķ����ʳ��ˮ������ԭ���������Ƶ� D��ʯ�;�������õ��������Ǵ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com