
����þ[Mg(ClO3)2]����������������ݼ��ȣ�ʵ�����Ʊ�����Mg(ClO3)2��6H2O���������£�
��֪����±����Ҫ�ɷ�ΪMgCl2��6H2O������MgSO4��FeCl2�����ʡ�
�����ֻ�������ܽ��(S)���¶�(T)�仯������ͼ��ʾ��
��1����������Ҫ����Ҫ���������� ��
��2������BaCl2��Ŀ���� ����MgO�����������������Ҫ�ɷ�Ϊ ��
��3������NaClO3������Һ������Ӧ�Ļ�ѧ����ʽΪ ���ٽ�һ����ȡMg(ClO3)2��6H2O��ʵ�鲽������Ϊ���������ᾧ���� ���� ���ܹ��ˡ�ϴ�ӡ�
��4����Ʒ��Mg(ClO3)2��6H2O�����IJⶨ��
����1��ȷ����3.50 g��Ʒ���100 mL��Һ��
����2��ȡ10.00 mL����ƿ�У�����10.00 mLϡ�����20 .00mL 1.000 mol��L��1��FeSO4��Һ���ȡ�
����3����ȴ�����£���0.100 mol��L��1 K2Cr2O7 ��Һ�ζ�ʣ���Fe2�����յ㣬�˹����з�Ӧ�����ӷ���ʽΪ��Cr2O72����6Fe2����14H����2Cr3����6Fe3����7H2O��
����4��������2��3�ظ����Σ�ƽ������K2Cr2O7 ��Һ15.00 mL��
��д������2�з�����Ӧ�����ӷ���ʽ�� ��
�ڲ�Ʒ��Mg(ClO3)2��6H2O����������Ϊ ��
��1��©�������������ձ� ��2����ȥSO42�� BaSO4��Fe(OH)3
��3��MgCl2��2NaClO3��Mg(ClO3)2��2NaCl�� ���ȹ��� ��ȴ�ᾧ
��4����ClO3�� ��6Fe2����6H����6Fe3����Cl����3H2O ��78.3%
���������������1����������Ҫ����Ҫ����������©�������������ձ�����2������BaCl2��Ŀ����ʹ����SO42-ת��Ϊ������ȥ����MgO������Һ��PH��4����ʱFe3+���γ�Fe(OH)3����.��ͬ����BaCl2����������ᱵ����һ����˳�ȥ�����Թ���������������Ҫ�ɷ�ΪBaSO4��Fe(OH)3����3������NaClO3������Һ������Ӧ�Ļ�ѧ����ʽΪMgCl2��2NaClO3��Mg(ClO3)2��2NaCl������Mg(ClO3)2����Һ�еõ�Mg(ClO3)2��6H2O���������ܽ�Ƚϴ����¶ȵ�Ӱ��仯�ϴ���ص��������ᾧ���ٳ��ȹ��ˣ������ȴ�ᾧ�õ�����4������������ԭ��Ӧ���������õ��ĵ����뻹ԭ��ʧȥ�ĵ�������������м��㡣��д������2�з�����Ӧ�����ӷ���ʽ��ClO3�� ��6Fe2����6H����6Fe3����Cl����3H2O������10.00 mL��Һ��1��0.02=3��2��0.015��0.1+ 6��n(ClO3�� ).���n(ClO3�� )="(" 0.011/6)mol.����3.50 g��Ʒ�к��е�Mg(ClO3)2��6H2O����Ϊ{( 0.011/6)mol��2}��10��299g/mol=2.741g.���Բ�Ʒ��Mg(ClO3)2��6H2O����������Ϊ(2.741g��3.50 g)��100��=78.3%
���㣺���鳣��������������ݼ�������þ[Mg(ClO3)2]����ȡ���ɷֲⶨ��֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧʵ���Ҳ����ķ�Һ�к���Fe3����Cu2����Ba2����Cl���������ӣ���������з����Է�Һ���д������Ի��ս������Ʊ��Ȼ������Ȼ������塣
��1������1�к��еĽ��������� ��
��2������ʱ����H2O2��Һ������Ӧ�����ӷ���ʽΪ ��
��3�����������У�������Ϊ�Լ�X���� ������ĸ����
| A��BaCl2 | B��BaCO3 |
| C��NaOH | D��Ba(OH)2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������һ������Ч���ˮ����������ҵ�ϳ�����NaClO��������������Ӧԭ��Ϊ��
���ڼ��������£�����NaClO����Fe(NO3)3�Ƶ�Na2FeO4
3NaClO + 2Fe(NO3)3 + 10NaOH��2Na2FeO4��+ 3NaCl + 6NaNO3 + 5H2O
��Na2FeO4��KOH��Ӧ����K2FeO4��Na2FeO4 + 2KOH��K2FeO4 + 2NaOH
��Ҫ�������������£�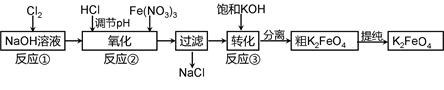
��1���������������ҺpHʱ����pH��ֽ���Բ���pH�Կ��Ƽ������������ʵ������pH��ֽ�ⶨ��ҺpH�IJ����� ��
��2������ͼ�С�ת��������Ӧ�ۣ�����ij�����½��еģ�˵�����¶���Ksp��K2FeO4�� Ksp��Na2FeO4���������������������
��3����Ӧ���¶ȡ�ԭ�ϵ�Ũ�Ⱥ���ȶԸ�����صIJ��ʶ���Ӱ�졣
ͼ1Ϊ��ͬ���¶��£�Fe(NO3)3��ͬ����Ũ�ȶ�K2FeO4�����ʵ�Ӱ�죻
ͼ2Ϊһ���¶��£�Fe(NO3)3����Ũ�����ʱ��NaClOŨ�ȶ�K2FeO4�����ʵ�Ӱ�졣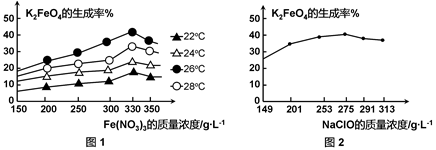
��ҵ����������¶�Ϊ �棬��ʱFe(NO3)3��NaClO������Һ�������Ũ��֮��Ϊ ��
��4��K2FeO4��ˮ��Һ���ס�ˮ�⡱��4FeO42- + 10H2O  4Fe(OH)3 + 8OH- + 3O2���ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ��Һ������ţ���
4Fe(OH)3 + 8OH- + 3O2���ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ��Һ������ţ���
| A��H2O | B��CH3COONa������� | C��NH4Cl������� | D��Fe(NO3)3������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʳ�����ճ�����ı���Ʒ��Ҳ����Ҫ�Ļ���ԭ�ϡ�
��ʳ�γ���������Ca2����Mg2����Fe3����SO42�����������ӣ�ʵ�����ṩ���Լ����£�����Na2CO3��Һ������K2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ��75%�Ҵ������Ȼ�̼��ʵ�����ᴿNaCl���������£�
��1������ȥ��Һ���е�Ca2����Mg2����Fe3����SO42�����ӣ�ѡ��A�������Ķ����Լ������μ�˳������Ϊi NaOH ii iii ���ѧʽ����
��2����д�������Լ�����ʱ������Ӧ�����ӷ�Ӧ����ʽ��
�����Լ�i�� ��
�����Լ�iii�� ��
��3��ϴ�ӳ�ȥNaCl������渽��������KCl��ѡ�õ��Լ� Ϊ �������ṩ���Լ���ѡ��
��4��ʵ�����õ�����������ʵ���Ũ��Ϊ0��400mol/L����ʵ����ijŨ�����Լ�ƿ�ϵ��й��������£�
��������Ũ��������ʵ������Ũ�ȵ�ϡ����480mL��
��������Ҫ�IJ��������� �����������ƣ�
������ȡ��Ũ��������Ϊ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ�÷��ڵ���ڵĺ�ɫ���壨���ܺ���MnO2��NH4Cl��ZnCl2�����ʣ���������ʵ�飺
��1���������������ж��õ���һ�ֲ���������_____________________________��
��2�������պ�Ĺ��������Թܣ��μ�˫��ˮ����Ѹ�ٲ���һ��ʹ�����ǵ�ľ����ȼ�����壬�ɴ��ƶϺ�ɫ�����к���MnO2�����ڷ�Ӧ�е�����Ϊ________��д���÷�Ӧ�Ļ�ѧ����ʽ_______________��MnO2��һ���������ԣ���д��һ��MnO2���������ķ�Ӧ�Ļ�ѧ����ʽ_____________________________________��
��3����֤����ڵ���Һ�к���NH4����������ijһʵ�鷽����¼Ƭ�ϡ�����д���пհף�
ʵ�������_______________________________________________________________��
ʵ��������ʹʪ��ĺ�ɫʯ����ֽ���������������
ʵ����ۣ���Һ����NH4�����ڡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪʵ������ȡ����ˮ��װ��ʾ��ͼ������ͼʾ�ش��������⡣
��1��ͼ�е��������ԵĴ����ǣ�________________________ ��_________________________________��
��2��B������������_______________��
��3��ʵ��ʱA�г�������������ˮ�⣬�����������_______����������__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʧȥ��ǩ��CaCl2��AgNO3��HCl����Na2CO3��ƿ��Һ��Ϊ��ȷ��������Һ�ijɷ֣������DZ��ΪA��B��C��D����л�ѧʵ�顣ʵ���¼���£�
| ʵ��˳�� | ʵ������ | ʵ������ |
| �� | A + B | ���������� |
| �� | B + D | ����ɫ��ζ����ų� |
| �� | C + B | �а�ɫ�������� |
| �� | A + D | �а�ɫ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ѧ��ȤС��ͬѧչ����Ư����������(NaClO2)���о���
ʵ��I����ȡNaClO2����
��֪��NaClO2������Һ���¶ȵ���38��ʱ����Ʒ����NaClO2��3H2O������38��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ��ʾװ�ý���ʵ�顣
��1��װ�â۵�������
װ�âٵ�������
��2��װ�â��в���ClO2�Ļ�ԭ����
װ�â����Ʊ�ClO2�Ļ�ѧ����ʽΪ
��3����װ�âܷ�Ӧ�����Һ���NaClO2����IJ�������Ϊ��
�ټ�ѹ��55�������ᾧ���ڳ��ȹ��ˣ��� ���ܵ���60�����õ���Ʒ��
ʵ��ⶨij����������Ʒ�Ĵ��ȡ�
�������ʵ�鷽����������ʵ�飺
��ȷ��ȡ��������������ƷС���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ(��֪��ClO2-+4I-+4H+=2H2O+2I2+Cl-)�������û��Һ���250mL������Һ��
����ȡ25��00mL������Һ����ƿ�У��Ӽ��ε�����Һ����c mol��L-1 Na2S2O3��Һ�ζ������ζ��յ㡣�ظ�2�Σ����ƽ��ֵΪV mL(��֪��I2+2S2O32-=2I-+S4O62-)��
��4���ﵽ�ζ��յ�ʱ������Ϊ
��5������Ʒ��NaClO2����������Ϊ (�ú�m��c��V�Ĵ���ʽ��ʾ)��
��6���ڵζ�������ȷ���������£���ʵ���ý��ƫ�ߣ�ԭ�������ӷ���ʽ��ʾΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��15�֣�ijУһ�о���ѧϰС��Ե����������ȷֽ�������ۡ�
�����Dz������ϵ�֪���������������ں����м���ʱ����79��134��,����ʧ��14��4����134��250�棬��ʧ��14��4����250��300�棬��ʧ��7��2����֮�������620�棬����ά�ֲ��䡣С�龭������ó���������300��620��Ĺ��������Ϊ��ˮ����ͭ��134��ʱ�Ĺ��������Ļ�ѧʽΪ ��
��С�����ˮ����ͭ�������¼��ȵĿ��ܱ仯���в��롣��������˲�������¼��ֲ��룺
�٣�CuO��SO3����
�ڣ�CuO��SO2��O2��
�ۣ�CuO��SO3��SO2��
�ܣ�CuO��SO3��SO2��O2
С�龭���������ۣ���Ϊ����۲���ʵ��Ϳ��ų������ǵ�������
�������ϣ���SO3Ϊ��ɫ���壬�۵�16��6�棬�е�44��8�档
��SO2���۵�:��72��4�棬�е�:��10�棻SO2ͨ��BaCl2��Һ�У�����������
��ʵ��̽����
С�鰴��ͼ��ʾ��װ��ʵ��װ�á�
��1����װ��װ�ú�δװҩƷǰ������еIJ����� ��
Dװ�õ������� ��
��2����ͼʾװ��ҩƷ���þƾ���ƶ���Ӳ���Թܼ��ȡ�һ�����B����Һ������ɫ���ǣ�C����Һ����ɫ��
����ʵ����������
��1��С��ͬѧ�����Ϊ��ˮ����ͭ�ȷֽ����Ӧ��Ϊ����ܡ�����һ��ͬѧ������ɣ�����ΪB����Һ������ɫ���Dz���һ����ȷ�������к���SO3�����������漰�Ļ�ѧ����ʽ�� �����ǣ�С��ͬѧ�����۾�����������һ��װ��E������Ϊ��װ��Ӧ���� ����װ����ĸ��֮�䡣����װ�ú�С������ʵ�飬֤���˲�����ȷʵ����SO3������Ϊ���Ǹ���ʲô����õ���һ���ۣ� ��
��2��С���������ˮ����ͭ�ȷֽ�Ļ�ѧ����ʽʱ���������ѡ����Ƿ��ָû�ѧ����ʽΪ��������ʽ��������������ƽ������������ܵط�����������ΪֻҪ��ȷ��ijЩ���ʵļ�����֮�ȣ�����ȷ���û�ѧ����ʽ������֪SO2��SO3�ļ�����֮�ȣ�����ȷ���û�ѧ����ʽ������SO2��SO3�ļ�����֮��Ϊx����д����ƽ��Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com