
�������зḻ��ʳƷ���������Դ��ҩ���ˮ����Դ��(����ͼ��ʾ) 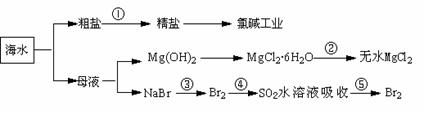
�����й�˵����ȷ����( )
A���ڢٲ��г�ȥ�����е�SO42-��Ca2+��Mg2+��Fe3+�����ʣ������ҩƷ˳��Ϊ��Na2CO3��Һ��NaOH��Һ��BaCl2��Һ�����˺������
B���ڢڲ��нᾧ����MgCl2��6H2O���ڿ��������ȷֽ�����ˮMgCl2
C���ڵڢۢܢݲ�����Ԫ�ؾ�������
D���ӵڢ۲����ڢݲ���Ŀ����Ϊ��Ũ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʹ0.1mol/L��NaHCO3��Һ��c(H+)��c(CO32��)��c(HCO3��)�����٣��䷽����
A��ͨ�������̼���� B�������������ƹ���
C��ͨ���Ȼ������� D�����뱥��ʯ��ˮ��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪I-��Fe2+��SO2��Cl-��H2O2���л�ԭ�ԣ�������������Һ�л�ԭ����˳��ΪSO2 > I- > Fe2+ > H2O2> Cl-,�����з�Ӧ�����ܷ������ǣ� ��
A��2Fe2++Cl2=2Fe3++2Cl-
B��2Fe3++SO2+2H2O��2Fe2++SO42-+4H+
C��H2O2+H2SO4=SO2��+O2��+2H2O
D��SO2+I2+2H2O��H2SO4+2HI
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС��̽��SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ������ͼ��ʾ��

��1��������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һע���� ��������װ�����������á�װ��E�������� ��װ��F��Ϊ ��Һ��
��2��װ��B������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ�� ��
a������ˮ b������Na2SO3��Һ
c������NaHSO3��Һ d������NaHCO3��Һ
��3��SO2���廹ԭFe3+��Ӧ�IJ�����________________�������ӷ��ţ���
��4��������װ����ͨ�������SO2Ϊ����֤C��SO2��Fe3+������������ԭ��Ӧ������ȡC�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м���KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ�����һ����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
�����ۣ����ڶ�����Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������____��ԭ����_______________________________________��
��5���ܱ���I���Ļ�ԭ������SO2��������__________________________��д���й����ӷ���ʽ��_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʯī���缫���1 mol/L CuSO4��Һ����c��Cu2����Ϊ0��5 mol/Lʱ��ֹͣ��⣬��ʣ����Һ�м������к������ʿ�ʹ�������Һ�ָ���ԭ��״��( )
A��CuSO4������������������ B��CuO
C��Cu��OH��2 D��CuSO4��5H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�˵����ȷ���ǣ� ��
A����0.2mol/L��NH3��H2O��0.1mol/L��HCl��Һ�������Ϻ�PH >7��
��c(Cl-)��c(NH4+)��c(OH-)��c(H+)
B����֪MgCO3��Ksp=6.82��10-6�������к��й���MgCO3����Һ�У�����
C��Mg2+��=C��CO32-�� ����C��Mg2+����C��CO32-��==6.82��10-6 mol•L��1
C�� 0.1mol/LNa2CO3��0.1mol/LNaHCO3��Һ�������ϣ�
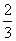 c(Na��)��c(CO32��)��c(HCO3��)��c(H2CO3)
c(Na��)��c(CO32��)��c(HCO3��)��c(H2CO3)
D���ö��Ե缫���Na2SO4��Һ������������������ʵ���֮��Ϊ2:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л�������G�Ǻϳ�ά������ҩ����м��壬��ṹ��ʽ��ͼ��ʾ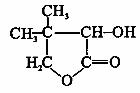 ��G�ĺϳ�·������ͼ��ʾ��
��G�ĺϳ�·������ͼ��ʾ��
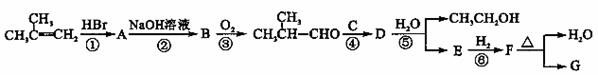
����A��F�ֱ����һ���л�������ϳ�·���в��ֲ��P��Ӧ��������ȥ
��֪��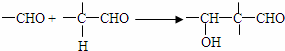
��ش��������⣺
��1��G�ķ���ʽ�� ��G�й����ŵ������� ��
��2���ڢڲ���Ӧ�Ļ�ѧ����ʽ�� ��
��3��B�����ƣ�ϵͳ�������� ��
��4���ڢڡ�����Ӧ������ȡ����Ӧ���� ������ţ���
��5���ڢܲ���Ӧ�Ļ�ѧ����ʽ�� ��
��6��д��ͬʱ��������������E������ͬ���칹��Ľṹ��ʽ ��
�� ֻ��һ�ֹ����ţ�����״�ṹ����-O-O-���ۺ˴Ź�������ֻ��2�ַ塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��(Ti )����Ϊ��������֮��ĵ�������������ͼ��ʾ�����ѳ����ȼ�ͼ״�����ɲ�ҵ�����Դ�������Դ�����ʣ����ٻ�����Ⱦ������д���пհף�
 |
��l����ⱥ��ʳ��ˮʱ���ܷ�Ӧ�����ӷ���ʽ�� ��
��2��������������д���������Ȼ����õ����Ȼ��ѵĻ�ѧ����ʽ
��
��3����֪����Mg(s) + Cl2(g)��MgCl2(s) ��H =��641kJ��mol-1
��Ti(s) + 2Cl2(g)��TiCl4(s) ��H = ��770kJ��mol-1
��2Mg(s) + TiCl4(s)��2MgCl2(s) + Ti(s) ��H�� ��
��Ӧ2Mg(s) + TiCl4(s) 2MgCl2(s) + Ti����Ar�����н��е������ǣ� ��
2MgCl2(s) + Ti����Ar�����н��е������ǣ� ��
��4���Լ״�������������������ҺΪԭ�ϣ�ʯīΪ�缫�ɹ���ȼ�ϵ�ء��õ���и����ϵĵ缫��Ӧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������ϡ�����У������ݲ��������ɵ�������ʹ����ʯ��ˮ����ǡ�������
���( )
A. ������ B����������� C��̼������� D. ����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com