
���û�ѧ��Ӧԭ���о�̼���������ȵȵ��ʼ��仯����ķ�Ӧ���������������������������������Ҫ�����塣
��1�����д�ʩ�У������ڻ����������� �����ţ���
a.��������ʹ�û�ʯȼ��
b.ʹ�������䡢�յ�
c.�ಽ�ж�˹�����������ר����˽�ҳ�
d.����ҵ��������������Һ������������ֱ���ŷ�
��2����ҵ�ϵġ���̼��ָ���Ǵӡ���������������������ü�Һ���ղ��õ�Ũ���Ķ�����̼�����ö�����̼�ϳɼ״���̼���ŵ��·���
��д�����ն�����̼�����ӷ���ʽ ��
�ڳ����£�0.1mol/LNaHCO3��Һ��pH��8������Һ��c(H2CO3) _______c(CO32��) �����������������������
�ۺϳɵļ״�������Ϊ����ȼ�ϵ�ص�ԭ�ϣ������Һ�Ǽ��Եģ����为���ĵ缫��ӦʽΪ ��
��3���������ȣ�ClO2����Ϊһ�ֻ���ɫ���壬�ǹ��ϵĸ�Ч�����װ�ȫ��ɱ������������ҵ���Ʊ�ClO2�ķ�Ӧԭ��Ϊ��4HC1(Ũ)+2NaClO3��2ClO2��+Cl2��+2H2O+2NaCl��������Ӧ�У�����1 mol ClO2����������HC1Ϊ ��
��4��SO2����ˮ���Եõ���Ԫ����H2SO3�������ᣩ��
��25��ʱ����NaOH��Һ������������ǡ���кͣ�����Һ�и�������Ũ�ȵĴ�С��ϵΪ ��
��25��ʱ����NaOH��Һ��H2SO3�����ʵ������ʱ�����ֻ��ҺpH��7�������Ҫ������ԭ�� ��
��14�֣�ÿ��2�֣���1��ad ��2����CO2��2OH����CO32-��H2O��CO2��OH����HCO3�� �ڣ�
��CH3OH��8OH�D�D6e����CO32����6H2O ��3��1mol��36.5g
��4����c(Na+)��c(SO32��)��c(OH��)��c(HSO3��)��c(H��)
��HSO3���ĵ���̶ȴ�����ˮ��̶Ȼ�HSO3����ˮ��̶�С�������̶�
���������������1��a����������ʹ�û�ʯȼ�ϣ��ɼ��ٷ�������Դ�������ڽ��ܼ��źͻ�����������a���� b�����ﰺ�ܲ�����ԭ�ӣ����dz����ֽ�Ĵ��������������ﰺ������ɫ�������䣬���Լ��ٳ�������ƻ�����b��ȷ��c������ȼ�յķ����о�����CO2��SO2��NOx�����壬�ಽ�л�˹�����������ר����˽�ҳ������Խ��ʹ����е�CO2��SO2��NOxŨ�ȣ���c��ȷ��d����ҵ�Ϸ����������ͷ�Һ�������ŷ������ˮ����Ⱦ�������ڻ�����������d����ѡad��
��2���ٶ�����̼���������������Ӧ�����κ�ˮ����Ӧ�����ӷ���ʽΪCO2+2OH����CO32-+H2O��CO2��OH����HCO3����
�ڳ����£�0.1mol/LNaHCO3��Һ��pH��8����˵��̼��������ӵ�ˮ����������̶ȣ�������Һ��c(H2CO3) ��c(CO32��)��
��ԭ����и���ʧȥ���ӷ���������Ӧ�������õ����ӷ�����ԭ��Ӧ�������Լ״�Ϊȼ������������ȼ�ϵ��������ͨ��O2������ͨ��״������ڵ��Һ�Ǽ�����Һ����˼״�������������CO32�������Ը����缫��ӦΪCH3OH��8OH�D�D6e����CO32����6H2O��
��3�����ݷ�Ӧ�Ļ�ѧ����ʽ��֪������������Ԫ�صĻ��ϼ۴ӣ�5�۽��͵���4�ۣ��õ�1�����ӣ���ClO2�ǻ�ԭ����Ȼ�������Ԫ�صĻ��ϼ۴ӣ�1�����ߵ�0�ۣ�ʧȥ1�����ӣ��������������������ÿ����1mol ClO2����������HC1Ϊ1mol��������1mol��36.5g/mol��36.5g��
��4����H2SO3��NaOHǡ���к�����Na2SO3����������������ǿ�������Σ����SO32��ˮ�⣬��Һ�Լ��ԣ��ҵ�һ��ˮ��ԶԶ���ڵڶ���ˮ�⣬��������ˮ�ⶼ����OH�����ӣ�������Һ������Ũ�ȴ�С��ϵ��c(Na+)��c(SO32��)��c(OH��)��c(HSO3��)��c(H��)��
��NaOH��Һ��H2SO3�����ʵ������ʱ����NaHSO3�����ҺpH��7�� ��NaHSO3��Һ�����ԣ���˵��HSO3���ĵ���̶ȴ�����ˮ��̶ȡ�
���㣺���鳣�����������Ⱦ����������ѧ��Դ���͵�أ�������ԭ��Ӧ�ļ��㣻����Ũ�ȴ�С�ıȽϣ������ʱ�Ķ����жϼ��й�ph�ļ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�о���ѧϰС��Ϊ��̽������ĵ������������������ʵ�顣
̽��Ũ�ȶԴ������̶ȵ�Ӱ��
��pH�Ʋⶨ25��ʱ��ͬŨ�ȵĴ����pH���������£�
| ����Ũ��(mol/L) | 0.0010 | 0.0100 | 0.0200 | 0.1000 | 0.2000 |
| pH | 3.88 | 3.38 | 3.23 | 2.88 | 2.73 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ�ֳ����Ļ������ҵ����Ҳ��һ����ȡ������ͭ�������ԭ�ϣ����з�ͭ����Ҫ����ΪFe�����Ʊ�����������������������̣�
pHֵ���ƿɲο���������
| ���� | ��ʼ����ʱ��pHֵ | ��ȫ����ʱ��pHֵ |
| �������� | 2.7 | 3.7 |
| ���������� | 7.6 | 9.6 |
| ������ͭ | 5.2 | 6.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��17�֣���ѧ��Ӧԭ���ڿ��к��������й㷺Ӧ�á�
��1��һ�������£�ģ��ij��ʯ�γɵķ�ӦaW+bQ��cN+dP+eR�õ�����ͼ��
�ٸ÷�Ӧ�ġ�H 0���>������=����<������
��ij�¶��£�ƽ�ⳣ������ʽΪK =c2��X��������ͼ��2���ж�X����������Ϊ____��
��2����E��F�����ܱ������У���һ�������·�����Ӧ��E��g��+F��s�� 2G��g��������
2G��g��������
���������ƽ��ʱG�����������%�����¶Ⱥ�ѹǿ�ı仯���±���ʾ��
��K��915�棩��K��810�棩�Ĺ�ϵΪK��915�棩____K��810�棩������ڡ��������ڡ���С�ڡ�����a��b��f���ߵĴ�С��ϵΪ ��1000�桢3��0 MPaʱE��ת����Ϊ____����3��25��ʱ��H2CO3 HCO3��+H+�ĵ��볣��Ka=4��10��7 mo1��L��1������¶��£�NaHCO3��ˮ�ⳣ��Kh= �������ʵ����Թ�ʵ��֤��Na2CO3��Һ�д���CO32��+H2O
HCO3��+H+�ĵ��볣��Ka=4��10��7 mo1��L��1������¶��£�NaHCO3��ˮ�ⳣ��Kh= �������ʵ����Թ�ʵ��֤��Na2CO3��Һ�д���CO32��+H2O  HCO3��+OH������ʵ ��
HCO3��+OH������ʵ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��15�֣���ҵ����ƽ�VOSO4�е�K2SO4��SiO2���ʳ�ȥ�����յõ�V2O5���������£�
��ش��������⣺
��1����������÷����ijɷ��� ��д��ѧʽ��������I������ ��
��2������ڡ��۵ı仯���̿ɼ�Ϊ����ʽR��ʾVO2+��HA��ʾ�л���ȡ������
R2(SO4)n (ˮ��)+ 2nHA���л��㣩 2RAn���л��㣩 + nH2SO4 (ˮ��)
2RAn���л��㣩 + nH2SO4 (ˮ��)
������ȡʱ��������������ԭ���� ��
����X�Լ�Ϊ ��
��3���ܵ����ӷ���ʽΪ ��
��4��25��ʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��֪��Cu(OH)2�Ƕ�Ԫ��������ᣨH3PO3���Ƕ�Ԫ���ᣬ��NaOH��Һ��Ӧ������Na2HPO3��
��1����ͭ����Һ��Cu2������ˮ�ⷴӦ�����ӷ���ʽΪ____���÷�Ӧ��ƽ�ⳣ��Ϊ____������֪��25��ʱ��Ksp[Cu(OH)2]��2.0��10��20mol3/L3��
��2������H3PO3�����ʿ��Ʋ�Na2HPO3ϡ��Һ��pH______7��������������������������£���10mL0.01mol/L H3PO3��Һ�еμ�10ml0.02mol/LNaOH��Һ����Һ�и�������Ũ���ɴ�С��˳����_________��
��3�����Na2HPO3��Һ�ɵõ������ᣬװ����ͼ��˵������Ĥֻ����������ͨ������Ĥֻ����������ͨ����
�������ĵ缫��ӦʽΪ____________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��17�֣������ҹ���ҵ��ˮƽ�IJ��Ϸ�չ�����ˮ��������Ⱦ�����Ϊ��Ҫ���⡣
��1������β���Ĵ����ŷ�����ɿ�����Ⱦ����Ҫ����֮һ����չȼ�ϵ������������Ч�ؽ���������⡣ֱ�Ӽ״�ȼ�ϵ��(DMFC)��������к��������ת��Ч�ʱ���ȼ��Ҫ��2��3������ؽṹ��ͼ��ʾ��c��ͨ�������ΪΪ______�����·�е��Ӵ�______��______(�A����B��)�ƶ���д����ظ����ĵ缫��Ӧ����ʽ 
��2����ҵ��ˮ�г�����һ������Cr2O72����������༰��̬ϵͳ�����ܴ�����ⷨ�Ǵ�������Ⱦ�ij��÷������÷���Fe���缫��⺬Cr2O72�������Է�ˮ�����ʱ�����������д����������ɣ�������Cr(OH)3��Fe(0H)3������
�ٷ�Ӧ�У�1molCr2O72����ȫ����Cr(OH)3���������·ͨ�����ӵ����ʵ���Ϊ_________ mol��
�ڳ����£�Cr(OH)3���ܶȻ� ����Cr3��Ũ��С��10
����Cr3��Ũ��С��10 mol
mol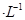 ʱ����Ϊ��ȫ�����������ȫ�����Һ��pH=6�������Һ���˺�Ϊ___________����ܡ���ֱ���ŷš�
ʱ����Ϊ��ȫ�����������ȫ�����Һ��pH=6�������Һ���˺�Ϊ___________����ܡ���ֱ���ŷš�
��3��������ˮ������ˮ�帻Ӫ��������NH4Cl��Һ�м�������NaOH���壬��Һ�� ________���������С�����䡱����25
________���������С�����䡱����25 ʱ��NH3?H2O�ĵ���ƽ�ⳣ��
ʱ��NH3?H2O�ĵ���ƽ�ⳣ�� �����¶���,1mol
�����¶���,1mol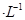 ��NH4Cl��Һ��
��NH4Cl��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijʳ�ð״����ɴ����봿ˮ���ƶ��ɣ����к͵ζ��ķ���ȷ�ⶨ���д�������ʵ���Ũ�ȡ�ʵ�鲽�裺������500mLŨ��ԼΪ0��1mol��L-1��NaOH��Һ������KHC8H4O4����Һȷ�ⶨ��NaOH��Һ��Ũ�ȣ�������֪ȷŨ�ȵ�NaOH��Һ�ⶨ�����Ũ�ȡ�
��1�����������NaOH�������ڴ��ձ��У�����500mL����ˮ�������ܽ⡣�����Ʋ��� ������С������С�����
��2������ʱNaOH�ڿ����м�����ˮ���������õ�NaOH��ҺŨ��ͨ����Ԥ�� ���С���������Dz���ֱ�����������Һ��ԭ��
��3�����İ״װ�װ�����Ậ��ԼΪ6g/100mL����������ʵ�Ũ��ԼΪ mol��L-1���ζ�ǰҪ�Ƚ��״�ϡ��10����ϡ�Ͱ״�ʱ��Ҫ��������100mL����ƿ���ձ����������� ��ͷ�ιܡ� ��
��4��ȷ��ȡϡ�ͺ�İ״�20��00mL������250mL��ƿ�У�����30mL����ˮ���ٵμӷ�ָ̪ʾ����������NaOH����Һ�ζ��� ��Ϊ�յ㡣
��5��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡ�״������Ϊ20��00mL��NaOH��ҺŨ��Ϊc mo1/L������ʵ ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 25��02 | 24��22 | 24��18 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ζ�ʵ���ǻ�ѧѧ������Ҫ�Ķ���ʵ�顣
��ش��������⣺
��1������к͵ζ������ñ�����ζ�δ֪Ũ�ȵ�NaOH��Һ�����в�����ɲⶨ���ƫ�ߵ��� (��ѡ����ĸ)
A���ζ��յ����ʱ�����ӵζ��̶ܿȣ�����������ȷ��
B��ʢװδ֪Һ����ƿ������ˮϴ����δ��δ֪Һ��ϴ
C����ʽ�ζ���������ˮϴ����δ�ñ�������ϴ
D���ζ�ǰ���ζ��ܼ��������ݣ��ζ���������ʧ
��2��������ԭ�ζ�����ȡ������Һ������ƿ�У���������ϡ���ᣬ��Ũ��Ϊ0.1mol��L��1�ĸ��������Һ�ζ��������ķ�ӦΪ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O�������м�¼��ʵ�����ݣ�
| �ζ����� | ����Һ��� (mL) | ��KMnO4��Һ���(mL) | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 25.00 | 0.50 | 20.40 |
| �ڶ��� | 25.00 | 3.00 | 23.00 |
| ������ | 25.00 | 4.00 | 24.10 |
| ������ | AgCl | AgBr | AgCN | Ag2CrO4 | AgSCN |
| ��ɫ | �� | dz�� | �� | ש�� | �� |
| Ksp | 1.77��10��10 | 5.35��10��13 | 1.21��10��16 | 1.12��10��12 | 1.0��10��12 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com