
��17�֣���ѧ��Ӧԭ���ڿ��к��������й㷺Ӧ�á�
��1��һ�������£�ģ��ij��ʯ�γɵķ�ӦaW+bQ��cN+dP+eR�õ�����ͼ��
�ٸ÷�Ӧ�ġ�H 0���>������=����<������
��ij�¶��£�ƽ�ⳣ������ʽΪK =c2��X��������ͼ��2���ж�X����������Ϊ____��
��2����E��F�����ܱ������У���һ�������·�����Ӧ��E��g��+F��s�� 2G��g��������
2G��g��������
���������ƽ��ʱG�����������%�����¶Ⱥ�ѹǿ�ı仯���±���ʾ��
��K��915�棩��K��810�棩�Ĺ�ϵΪK��915�棩____K��810�棩������ڡ��������ڡ���С�ڡ�����a��b��f���ߵĴ�С��ϵΪ ��1000�桢3��0 MPaʱE��ת����Ϊ____����3��25��ʱ��H2CO3 HCO3��+H+�ĵ��볣��Ka=4��10��7 mo1��L��1������¶��£�NaHCO3��ˮ�ⳣ��Kh= �������ʵ����Թ�ʵ��֤��Na2CO3��Һ�д���CO32��+H2O
HCO3��+H+�ĵ��볣��Ka=4��10��7 mo1��L��1������¶��£�NaHCO3��ˮ�ⳣ��Kh= �������ʵ����Թ�ʵ��֤��Na2CO3��Һ�д���CO32��+H2O  HCO3��+OH������ʵ ��
HCO3��+OH������ʵ ��
��1���٣�����R����2�����ڣ�f��a��b��69.5%����3��2.5��10��8��ȡ����̼������Һ�����Թ��У��μ��Ȼ�����Һ�а�ɫ�������ɣ�֤������̼�������ȡ����̼������Һ�����Թ��У��μӷ�̪��Һ��죬֤�������������ɡ�
���������������1���ٸ���ͼ��1��֪���÷�Ӧ��K�����¶ȵ����߶�������÷�Ӧ����Ϊ���ȷ�Ӧ����H��0���ڸ���ͼ��2��֪���÷�Ӧ�Ļ�ѧ����ʽΪ2W+Q��3N+P+2R��ij�¶��£�ƽ�ⳣ������ʽΪK =c2��X��������ƽ�ⳣ������ʽ����дԭ�����Һ�岻д��ƽ�ⳣ������ʽ������ͼ��2���ж�X����������ΪR��
��2����Ӧ��E��g��+F��s�� 2G��g������Ϊ���������С�ķ�Ӧ����ѹ��ƽ�������ƶ���G�����������%����С�����������ݷ���������Ϊѹǿ��ƽ��Ӱ������ݣ���54.0��a��b��c��75.0��d��e��f��82.0���ɵ�a��b��f���ߵĴ�С��ϵΪf��a��b��������������֪��e��c��54.0,������������Ϊ�¶ȶ�ƽ���Ӱ�죬���£�G�����������%������ƽ�������ƶ�����÷�Ӧ����Ϊ���ȷ�Ӧ����K��915�棩��K��810�棩�Ĺ�ϵΪK��915�棩����K��810�棩�������������֪��1000�桢3��0 MPaʱG�����������%��Ϊ82.0�����ݷ�Ӧ��E��g��+F��s��
2G��g������Ϊ���������С�ķ�Ӧ����ѹ��ƽ�������ƶ���G�����������%����С�����������ݷ���������Ϊѹǿ��ƽ��Ӱ������ݣ���54.0��a��b��c��75.0��d��e��f��82.0���ɵ�a��b��f���ߵĴ�С��ϵΪf��a��b��������������֪��e��c��54.0,������������Ϊ�¶ȶ�ƽ���Ӱ�죬���£�G�����������%������ƽ�������ƶ�����÷�Ӧ����Ϊ���ȷ�Ӧ����K��915�棩��K��810�棩�Ĺ�ϵΪK��915�棩����K��810�棩�������������֪��1000�桢3��0 MPaʱG�����������%��Ϊ82.0�����ݷ�Ӧ��E��g��+F��s�� 2G��g����������ʽ���㣬����ʼ�����E���ʵ���Ϊ1mol��ת����Ϊx��
2G��g����������ʽ���㣬����ʼ�����E���ʵ���Ϊ1mol��ת����Ϊx��
E��g��+F��s�� 2G��g��
2G��g��
��ʼ��mol����1 ���� 0
ת����mol����x 2x
ƽ�⣨mol����1��x 2x
��2x/1+x=82.0%�����x=0.695��E��ת����Ϊ69.5%��
��3��25��ʱ��H2CO3���볣��Ka=c(HCO3��)c(H��)/c(H2CO3)��NaHCO3��ˮ�ⳣ��Kh=c(H2CO3)c(OH��)/c(HCO3��)����Ka��Kh=Kw��Kh=Kw/Ka=10��14/4��10��7=2.5��10��8��֤��Na2CO3��Һ�д��� CO32��+H2O HCO3��+OH������ʵΪȡ����̼������Һ�����Թ��У��μ��Ȼ�����Һ�а�ɫ�������ɣ�֤������̼�������ȡ����̼������Һ�����Թ��У��μӷ�̪��Һ��죬֤�������������ɡ�
CO32��+H2O HCO3��+OH������ʵΪȡ����̼������Һ�����Թ��У��μ��Ȼ�����Һ�а�ɫ�������ɣ�֤������̼�������ȡ����̼������Һ�����Թ��У��μӷ�̪��Һ��죬֤�������������ɡ�
���㣺���黯ѧƽ��ͼ���ƽ����㣬��������Ի�ѧƽ���Ӱ�죬������ʵĵ���������ˮ��ƽ�⡣


 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ˮ���ڵ���ƽ�⣬H2O H++OH? ����ش�
H++OH? ����ش�
���������¶ȣ�����̶Ƚ� ����Һ�� �ԡ�
�������м�������ϡ��ˮ��ˮ�ĵ���ƽ�� �ƶ�����Һ��PH ��
���������м�������NaHSO4��Һ��ˮ�����ӻ����� ����ˮ�������c(H+) c(OH_)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���仯�����й㷺��Ӧ�ã���SO2���ʵ��о��Ǹ��л�ѧ��ѧ��һ����Ҫ���ݡ�
I���Ա��о���һ����Ҫ���о�������������ĵ��ʼ����ֻ����ﰴ���±���ʾ�ֳ�3 �飬���2��������M�Ļ�ѧʽ�� ��
| ��1�� | ��2�� | ��3�� |
| S (����) | SO2��H2SO3��M��NaHSO3 | SO3��H2SO4��Na2SO4��NaHSO4 |

| n(SO32��)��n(HSO3��) | 91��9 | 1��1 | 9��91 |
| pH | 8.2 | 7.2 | 6.2 |
| ��� | ʵ�鷽�� | ʵ������ | ���� |
| ������ | ����һ����Һ�м���KMnO4��Һ��Һ | �Ϻ�ɫ��ȥ | SO2��Fe3+��Ӧ������Fe2+ |
| ������ | ���ڶ�����Һ�м��� | | SO2��Fe3+��Ӧ������Fe2+ |
| ������ | ���ڶ�����Һ�м��� | | SO2��Fe3+��Ӧ������SO42�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ǿ�ᣬ��ѧ�ν�������ˮ��Һ�п�����ȫ���롣����ʵ�ǣ�������ˮ�еĵ�һ����������ȫ�ģ��ڶ������벢����ȫ����������Ϊ:H2SO4=H++HSO4-��HSO4- H+ + S042-��
H+ + S042-��
��ش������й�����:
��1��Na2SO4��Һ��_(������ԡ��������ԡ��������ԡ�)����������_
(�����ӷ���ʽ��ʾ)��
��2��H2SO4��Һ��BaC12��Һ��Ӧ�����ӷ���ʽΪ_ ��
��3����0��l0mol��L-1��Na2SO4��Һ�У���������Ũ�ȹ�ϵ��ȷ����_ (��д���)��
| A��c(Na+)=c(SO42-)+c��HSO4һ)+c(H2SO4) |
| B��c(OH-)="c(" HSO4-)+c(H+) |
| C��c( Na+)+c(H+)=c(OH-)+c(HSO4-)+2c(SO42-) |
| D��c( Na+)=2c(SO42-)+2c(HSO4-) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ᣨH3PO3���Ƕ�Ԫ�ᣬH3PO3��Һ���ڵ���ƽ�⣺H3PO3 H+ + H2PO3����������������NaOH��Һ��Ӧ������Na2HPO3��
H+ + H2PO3����������������NaOH��Һ��Ӧ������Na2HPO3��
��1����д��������������NaOH��Һ��Ӧ�����ӷ���ʽ_____________________________________��
��ij�¶��£�0.1000 mol��L��1��H3PO3��ҺpH�Ķ���Ϊ1.6������ʱ��Һ��c (H+) = 2.5��10��2 mol��L��1����OH��֮���������ӵ�Ũ����С�����˳���� �����¶���H3PO3����ƽ���ƽ�ⳣ��K= ����H3PO3�ڶ���������Բ��ƣ����������λ��Ч���֣�
����H3PO3��Һ�еμ�NaOH��Һ�����ԣ�������Һ��c��Na+��_______ c��H2PO3-��+ 2c��HPO32-�����>���� ��<�� ��=������
��2�����������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽ_______________________��
��3�����Na2HPO3��ҺҲ�ɵõ������ᣬװ��ʾ��ͼ���£�
˵������Ĥֻ����������ͨ������Ĥֻ����������ͨ����
�������ĵ缫��ӦʽΪ_____________________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
[14��]��֪��I2��2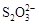

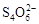 ��2I�D��������ʵ��ܶȻ��������±���
��2I�D��������ʵ��ܶȻ��������±���
| ���� | Cu(OH)2 | Fe(OH)3 | CuCl | CuI |
| Ksp | 2.2��10��20 | 2.6��10��39 | 1.7��10��7 | 1.3��10��12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
���û�ѧ��Ӧԭ���о�̼���������ȵȵ��ʼ��仯����ķ�Ӧ���������������������������������Ҫ�����塣
��1�����д�ʩ�У������ڻ����������� �����ţ���
a.��������ʹ�û�ʯȼ��
b.ʹ�������䡢�յ�
c.�ಽ�ж�˹�����������ר����˽�ҳ�
d.����ҵ��������������Һ������������ֱ���ŷ�
��2����ҵ�ϵġ���̼��ָ���Ǵӡ���������������������ü�Һ���ղ��õ�Ũ���Ķ�����̼�����ö�����̼�ϳɼ״���̼���ŵ��·���
��д�����ն�����̼�����ӷ���ʽ ��
�ڳ����£�0.1mol/LNaHCO3��Һ��pH��8������Һ��c(H2CO3) _______c(CO32��) �����������������������
�ۺϳɵļ״�������Ϊ����ȼ�ϵ�ص�ԭ�ϣ������Һ�Ǽ��Եģ����为���ĵ缫��ӦʽΪ ��
��3���������ȣ�ClO2����Ϊһ�ֻ���ɫ���壬�ǹ��ϵĸ�Ч�����װ�ȫ��ɱ������������ҵ���Ʊ�ClO2�ķ�Ӧԭ��Ϊ��4HC1(Ũ)+2NaClO3��2ClO2��+Cl2��+2H2O+2NaCl��������Ӧ�У�����1 mol ClO2����������HC1Ϊ ��
��4��SO2����ˮ���Եõ���Ԫ����H2SO3�������ᣩ��
��25��ʱ����NaOH��Һ������������ǡ���кͣ�����Һ�и�������Ũ�ȵĴ�С��ϵΪ ��
��25��ʱ����NaOH��Һ��H2SO3�����ʵ������ʱ�����ֻ��ҺpH��7�������Ҫ������ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ѧ�������֮����ת�����˵�����ʵ��������أ�������������������Ҫ��Ӧ�ã�ͬʱҲ��ѧ���γɻ�ѧѧ����������Ҫ��ɲ��֡�
��1������״̬�£��Ƶĵ��ʺ��Ȼ���������ɿɳ����(��ͼ1)����Ӧԭ��Ϊ��2Na��FeCl2 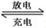 Fe��2NaCl,�õ�طŵ�ʱ��������ӦʽΪ ________________ _____��
Fe��2NaCl,�õ�طŵ�ʱ��������ӦʽΪ ________________ _____��
���ʱ��__________(д��������)�缫�ӵ�Դ�ĸ�����
�õ�صĵ����Ϊ________ _��
��2��ijͬѧ��ͭƬ��ʯī���缫���һ��Ũ�ȵ�����ͭ��Һ(��ͼ2)��һ��ʱ��ֹͣͨ��ȡ���缫�����ڵ������Һ�м���0.98g������ͭ��ĩǡ����ȫ�ܽ⣬���ⶨ������Һ����ǰ��ȫ��ͬ����ش��������⣺
��Y�缫������ ������ (�������ԭ��)��Ӧ��
�ڵ�������X�缫�Ϸ����ĵ缫����Ӧʽ�ǣ�
�����ڵ������Һ�м���������С�մ�ַ�Ӧ����������ڱ�״������ռ�������
��3������ʱ��BaSO4��Ksp��1.08��10-10,�ֽ��������BaCl2��Һ��2.0��10-3mol/l��Na2SO4
��Һ��ϡ���Ҫ����BaSO4������BaCl2��Һ����СŨ��Ϊ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
�ٽ���ʽ�ζ���������ˮϴ����������Һ��ϴ����ע�������Һ�����ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦��"0"�̶����µ�λ�ã����¶���������ƿ������ˮϴ�����ô�����Һ��ϴ��ƿ2��3�Σ��Ӽ�ʽ�ζ����з���20.00mL������Һ����ƿ�С�
�ڽ���ʽ�ζ���������ˮϴ�������ñ���Һ��ϴ2-3�κ�������ע��0.1000 mol/L�����ᣬ���ڵζ��ܵļ��첿�ֳ�����Һ����ʹҺ�洦��"0"�̶����µ�λ�ã����¶�����
������ƿ�е����̪��ָʾ�������еζ����ζ���ָʾ���պñ�ɫ���Ұ��������ɫ���ٸı�Ϊֹ�����������������ΪV1mL��
���ظ����Ϲ��̣����ڵζ�����������ƿ����5mL������ˮ�����������������ΪV2 mL���Իش��������⣺
��1����ƿ�е���Һ�� ɫ��Ϊ�� �� ɫʱ��ֹͣ�ζ���
��2����С���ڲ�����еĴ����� ��
�ɴ���ɵIJⶨ��� (ƫ�ߡ�ƫ�ͻ���Ӱ��)��
��3����ͼ����ij�εζ�ʱ�ĵζ����е�Һ�棬�����Ϊ mL��
��4�������������ݣ�
| �ζ����� | ����Һ���(mL) | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 20.00 | 0.50 | 22.60 |
| �ڶ��� | 20.00 | 1.00 | 24.50 |
| ������ | 20.00 | 2.10 | 24.00 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com