
�����ᣨH3PO3���Ƕ�Ԫ�ᣬH3PO3��Һ���ڵ���ƽ�⣺H3PO3 H+ + H2PO3����������������NaOH��Һ��Ӧ������Na2HPO3��
H+ + H2PO3����������������NaOH��Һ��Ӧ������Na2HPO3��
��1����д��������������NaOH��Һ��Ӧ�����ӷ���ʽ_____________________________________��
��ij�¶��£�0.1000 mol��L��1��H3PO3��ҺpH�Ķ���Ϊ1.6������ʱ��Һ��c (H+) = 2.5��10��2 mol��L��1����OH��֮���������ӵ�Ũ����С�����˳���� �����¶���H3PO3����ƽ���ƽ�ⳣ��K= ����H3PO3�ڶ���������Բ��ƣ����������λ��Ч���֣�
����H3PO3��Һ�еμ�NaOH��Һ�����ԣ�������Һ��c��Na+��_______ c��H2PO3-��+ 2c��HPO32-�����>���� ��<�� ��=������
��2�����������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽ_______________________��
��3�����Na2HPO3��ҺҲ�ɵõ������ᣬװ��ʾ��ͼ���£�
˵������Ĥֻ����������ͨ������Ĥֻ����������ͨ����
�������ĵ缫��ӦʽΪ_____________________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ_____________________��
��14�֣���1�� �� H3PO3+OH����H2PO3��+H2O ��2�֣�
��c��HPO32-��< c��H2PO3-��< c��H+�� ��2�֣�8.3��10��3mol/L ��2�֣� �� ����2�֣�
��2��H3PO3 + I2 +H2O = 2HI+ H3PO4 ��2�֣� ��3���� 2H+ + 2e����H2�� ��2�֣�
��HPO32��+ 2H+��H3PO3��2�֣���HPO32��+ H+��H2PO3����H2PO3��+ H+��H3PO3����1�֣�
���������������1�����������Ƕ�Ԫ�ᣬ������������������Ʒ�Ӧ����NaH2PO3��H2O�����Ը÷�Ӧ����ʽΪH3PO3+OH����H2PO3��+H2O��
��0.1000mol?L-1��H3PO3��ҺpH�Ķ���Ϊ1.6��������Ũ��С��������Ũ�ȣ������������Ƕ�Ԫ���ᣬ��ˮ��Һ�зֲ����룬�ҵ�һ������̶ȴ��ڵڶ��������������ж������������ɣ�����������Ũ������������Ũ�ȴ�С˳����c��HPO32-��< c��H2PO3-��< c��H+����
H3PO3  H+ + H2PO3��
H+ + H2PO3��
��ʼʱ������Ũ�ȣ�mol?L��1�� 0.10 0 0
��Ӧ�ĸ����ʵ�Ũ�ȣ�mol?L��1��2.5��10��2 2.5��10��2 2.5��10��2
ƽ��ʱ�����ʵ�Ũ�ȣ�mol?L��1��0.10��2.5��10��2 2.5��10��2 2.5��10��2
K��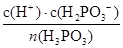 ��
��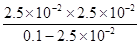 ��8.3��10��3mol/L
��8.3��10��3mol/L
����Һ�����ԣ���c��H+����c��OH-��������Ϊ��Һ�ʵ����ԣ���c��Na+��+C��H+����C��OH-��+c��H2PO3-��+2c��HPO32-��������C��H+��=C��OH-��������c��Na+����c��H2PO3-��+2c��HPO32-����
��2�������ǿ�����ԣ����������ǿ��ԭ�ԣ�����������͵��ܷ���������ԭ��Ӧ�������������ᣬ��Ӧ�Ļ�ѧ����ʽΪH3PO3 + I2 +H2O = 2HI+ H3PO4 ��
��3���ٵ����������õ����ӣ�������ԭ��Ӧ�������������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H+ + 2e����H2����
�ڲ�Ʒ����HPO32-�������ӽ�����������ᣬ��˷�Ӧ���ӷ���ʽΪHPO32��+ 2H+��H3PO3��
���㣺������ʵĵ��롢����ƽ�ⳣ���ļ��㡢��Һ��������Ũ�ȴ�С�Ƚϡ�������ԭ��Ӧ����ʽ����д�Լ��绯ѧԭ����Ӧ�õ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������£�����ˮ��Һ����Sn2+��Sn4+������Ҫ������ʽ��SnSO4��һ����Ҫ�������Σ��㷺Ӧ���ڶ�����ҵ�����Ʊ�·�����£�
�ش��������⣺
��1��SnCl2�����������ˮֱ���ܽ��ԭ����
�������۵�������
��2����ӦI���ɵij���ΪSnO��д���÷�Ӧ�Ļ�ѧ����ʽ��
��3����������Ѿ���ϴ�ӡ��ɾ��IJ����ǣ� ��3�֣�
��4����Ӧ�����������֮һ�ǿ�����Һ��pH������Һ��c(Sn2+)=1.0mol��L��1����������Ӧ������ҺpH ������֪��Ksp[Sn(OH)2]=1.0��10��26��
��5�����������£�SnSO4��������˫��ˮ��ȥ��������д����������Ӧ�����ӷ���ʽ��
��
��6����ʪ�����У�����ͭ��ʹ��������Ҳ�ܷ�ֹ�γ�ͭ�̣������йص�ԭ��������ԭ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(һ)��1������Ҳ��һ�����ȼ�ϣ�������ȫȼ��ʱ��Ч�ʽ��Ͳ�������ж����������Ⱦ��
��֪�� CH4(g) + 2O2(g) �� CO2(g) + 2H2O(l) ��H1���D890.3 kJ/mol
2CO (g) + O2(g) �� 2CO2(g) ��H2���D566.0 kJ/mol
����鲻��ȫȼ������һ����̼��Һ̬ˮʱ����Ч��ֻ����ȫȼ��ʱ��________��������������1λС������
��2������ȼ�ϵ�ؿ����������������ʡ���ͼ�����ü���ȼ�ϵ�ص��50 mL 2 mol/L���Ȼ�ͭ��Һ��װ��ʾ��ͼ��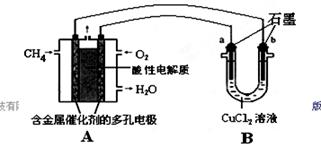
��ش�
�ټ���ȼ�ϵ�صĸ�����Ӧʽ��________��
�ڵ���·����0.1 mol����ͨ��ʱ��________���a����b����������________g��
�������±��Ǽ���������ʵĵ���ƽ�ⳣ�������ܵ���ʵ�
�ܶȻ�Ksp (25��)��
| ����� | ƽ�ⷽ��ʽ | ƽ�ⳣ��K | Ksp |
| CH3COOH | CH3COOH CH3COO-��H+ CH3COO-��H+ | 1.76��10-5 | |
| H2CO3 | H2CO3 H+��HCO3- H+��HCO3-HCO3-  H+��CO32- H+��CO32- | K1��4.31��10-7 K2��5.61��10-11 | |
| C6H5OH | C6H5OH  C6H5O-��H+ C6H5O-��H+ | 1.1��10-10 | |
| H3PO4 | H3PO4 H+��H2PO4- H+��H2PO4-H2PO4-  H+��HPO32- H+��HPO32-HPO42-  H+��PO43- H+��PO43- | K1��7.52��10-3 K2��6.23��10-8 K3��2.20��10-13 | |
| NH3��H2O | NH3��H2O NH4+��OH- NH4+��OH- | 1.76��10-5 | |
| BaSO4 | BaSO4 Ba2+��SO42- Ba2+��SO42- | | 1.07��10-10 |
| BaCO3 | BaCO3 Ba2+��CO32- Ba2+��CO32- | | 2.58��10-9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ�ֳ����Ļ������ҵ����Ҳ��һ����ȡ������ͭ�������ԭ�ϣ����з�ͭ����Ҫ����ΪFe�����Ʊ�����������������������̣�
pHֵ���ƿɲο���������
| ���� | ��ʼ����ʱ��pHֵ | ��ȫ����ʱ��pHֵ |
| �������� | 2.7 | 3.7 |
| ���������� | 7.6 | 9.6 |
| ������ͭ | 5.2 | 6.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���Ի�ͭ��(��Ҫ�ɷ���CuFeS2�����ʲ�����ˮ����)Ϊԭ�ϣ��Ʊ���ɫ����G���仯ѧʽΪ[Cu(NH3)4]SO4��H2O���漰�������£�
��֪25��ʱ�����ֽ�������������ܶȻ���������ȫ������pH��Χ���±���
| | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| Ksp | 8.0��10-16 | 2.2��10-22 | 4.0��10-38 |
| ��ȫ����pH | ��9.6 | ��6.4 | ��3.2 |
 [Cu(NH3)4]2++2OH-+4H2O��д���÷�Ӧ��ƽ�ⳣ������ʽ�� ��
[Cu(NH3)4]2++2OH-+4H2O��д���÷�Ӧ��ƽ�ⳣ������ʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��17�֣���ѧ��Ӧԭ���ڿ��к��������й㷺Ӧ�á�
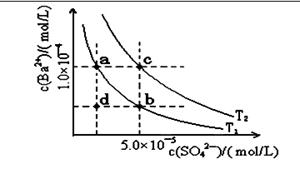
��1��һ�������£�ģ��ij��ʯ�γɵķ�ӦaW+bQ��cN+dP+eR�õ�����ͼ��
�ٸ÷�Ӧ�ġ�H 0���>������=����<������
��ij�¶��£�ƽ�ⳣ������ʽΪK =c2��X��������ͼ��2���ж�X����������Ϊ____��
��2����E��F�����ܱ������У���һ�������·�����Ӧ��E��g��+F��s�� 2G��g��������
2G��g��������
���������ƽ��ʱG�����������%�����¶Ⱥ�ѹǿ�ı仯���±���ʾ��
��K��915�棩��K��810�棩�Ĺ�ϵΪK��915�棩____K��810�棩������ڡ��������ڡ���С�ڡ�����a��b��f���ߵĴ�С��ϵΪ ��1000�桢3��0 MPaʱE��ת����Ϊ____����3��25��ʱ��H2CO3 HCO3��+H+�ĵ��볣��Ka=4��10��7 mo1��L��1������¶��£�NaHCO3��ˮ�ⳣ��Kh= �������ʵ����Թ�ʵ��֤��Na2CO3��Һ�д���CO32��+H2O
HCO3��+H+�ĵ��볣��Ka=4��10��7 mo1��L��1������¶��£�NaHCO3��ˮ�ⳣ��Kh= �������ʵ����Թ�ʵ��֤��Na2CO3��Һ�д���CO32��+H2O  HCO3��+OH������ʵ ��
HCO3��+OH������ʵ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��15�֣���ҵ����ƽ�VOSO4�е�K2SO4��SiO2���ʳ�ȥ�����յõ�V2O5���������£�
��ش��������⣺
��1����������÷����ijɷ��� ��д��ѧʽ��������I������ ��
��2������ڡ��۵ı仯���̿ɼ�Ϊ����ʽR��ʾVO2+��HA��ʾ�л���ȡ������
R2(SO4)n (ˮ��)+ 2nHA���л��㣩 2RAn���л��㣩 + nH2SO4 (ˮ��)
2RAn���л��㣩 + nH2SO4 (ˮ��)
������ȡʱ��������������ԭ���� ��
����X�Լ�Ϊ ��
��3���ܵ����ӷ���ʽΪ ��
��4��25��ʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��17�֣������ҹ���ҵ��ˮƽ�IJ��Ϸ�չ�����ˮ��������Ⱦ�����Ϊ��Ҫ���⡣
��1������β���Ĵ����ŷ�����ɿ�����Ⱦ����Ҫ����֮һ����չȼ�ϵ������������Ч�ؽ���������⡣ֱ�Ӽ״�ȼ�ϵ��(DMFC)��������к��������ת��Ч�ʱ���ȼ��Ҫ��2��3������ؽṹ��ͼ��ʾ��c��ͨ�������ΪΪ______�����·�е��Ӵ�______��______(�A����B��)�ƶ���д����ظ����ĵ缫��Ӧ����ʽ 
��2����ҵ��ˮ�г�����һ������Cr2O72����������༰��̬ϵͳ�����ܴ�����ⷨ�Ǵ�������Ⱦ�ij��÷������÷���Fe���缫��⺬Cr2O72�������Է�ˮ�����ʱ�����������д����������ɣ�������Cr(OH)3��Fe(0H)3������
�ٷ�Ӧ�У�1molCr2O72����ȫ����Cr(OH)3���������·ͨ�����ӵ����ʵ���Ϊ_________ mol��
�ڳ����£�Cr(OH)3���ܶȻ� ����Cr3��Ũ��С��10
����Cr3��Ũ��С��10 mol
mol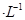 ʱ����Ϊ��ȫ�����������ȫ�����Һ��pH=6�������Һ���˺�Ϊ___________����ܡ���ֱ���ŷš�
ʱ����Ϊ��ȫ�����������ȫ�����Һ��pH=6�������Һ���˺�Ϊ___________����ܡ���ֱ���ŷš�
��3��������ˮ������ˮ�帻Ӫ��������NH4Cl��Һ�м�������NaOH���壬��Һ�� ________���������С�����䡱����25
________���������С�����䡱����25 ʱ��NH3?H2O�ĵ���ƽ�ⳣ��
ʱ��NH3?H2O�ĵ���ƽ�ⳣ�� �����¶���,1mol
�����¶���,1mol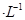 ��NH4Cl��Һ��
��NH4Cl��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�
(1)�ñ�����ζ����������������Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע�� ��ֱ������һ���������Һ��ɫ�ɻ�ɫ��Ϊ��ɫ���� Ϊֹ��
(2)���в����п���ʹ��������������Һ��Ũ��ֵƫ�͵��� ��
| A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������� |
| B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����δ���� |
| C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ |
| D����ȡ�������ʱ����ʼ���Ӷ������ζ��������Ӷ��� |

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com