
| C-O | C=O | C��O | |
| CO | 357.7 | 798.9 | 1071.9 |
| N-N | N=N | N��N | |
| N2 | 154.8 | 418.4 | 941.7 |
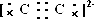 ��CaC2�ɸ����Ӻ�C22-���ɣ�����ʽΪ��
��CaC2�ɸ����Ӻ�C22-���ɣ�����ʽΪ��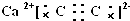 ��
��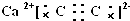 ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Na2CO3��KOH��CaO |
| B��CO��NaOH��KCl |
| C��H2O��H2SO4��NaCl |
| D��CO2��KOH��FeO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ƺͼطֱ�����ˮ��Ӧ���жϽ������ǿ�� |
| B��Br2��I2�ֱ���������H2��Ӧ���ж������ķǽ������ǿ�� |
| C��̼������Һ�Լ��ԣ���������Һ�����ԣ��ж�����̼�ķǽ������ǿ�� |
| D����MgSO4��Al��NO��3��Һ�зֱ��������İ�ˮ���ж�þ�����Ľ������ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| ������ | NaF | MgF2 | SiF4 |
| �۵�/K | 1266 | 1534 | 183 |
| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

 ���ɣ���ṹ��ʽ��
���ɣ���ṹ��ʽ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com