
����½�1g������C2H2�ڿ�������ȫȼ�գ�����CO2�����Һ̬ˮ���ų�akJ���������ʾC2H2ȼ���ȵ��Ȼ�ѧ����ʽΪ��_______________________
�ƣ���4�֣�����ƽ�����ʢ��ǿ��ԭ��Һ̬�£�N2H4����ǿ������Һ̬˫��ˮ�������ǻ�Ϸ�Ӧʱ��������������������ˮ���������ų��������ȡ���֪0.4molҺ̬����������Һ̬˫��ˮ��Ӧ�����ɵ�����ˮ�������ų�256KJ����������Ӧ���Ȼ�ѧ����ʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ��������±���ʾ��
| Ԫ�� | �� | �� | �� | �� |
| ԭ�Ӱ뾶/nm | 0.186 | 0.102 | 0.152 | 0.074 |
| ��Ҫ���ϼ� | +1 | +6��¯2 | +1 | ¯2 |
������������ȷ����
A�����ʵ��۵�ȱ����ʵ��۵��
B�����ʿ��õ���������εķ���ұ������
C�������£����������⻯���ΪҺ��
D���ҵļ�����������Ԫ�ظ����γɵļ������а뾶����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ˮϡ��0.1 mol/L����ʱ��ʼ�ձ����������Ƶ��� (����)
A����Һ�е�c(CH3COO��) B����Һ�е�c(H��)
C������ĵ���ƽ�ⳣ�� D����Һ�е�c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��V�����ܱ������н������·�Ӧ����mAʮnB  pCʮqD����A���ʱ�ʾ��ƽ����Ӧ����Ϊ
pCʮqD����A���ʱ�ʾ��ƽ����Ӧ����Ϊ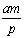 Ħ��(������)����t����ʱ,D�������ӵ����ʵ����ǣ�
Ħ��(������)����t����ʱ,D�������ӵ����ʵ����ǣ�
A��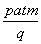 Ħ B��
Ħ B��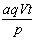 Ħ C��
Ħ C��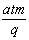 Ħ D��
Ħ D��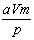 Ħ
Ħ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ȼ�ѧ����ʽ��ȷ����
A�������ȼ����Ϊ890.3 kJ��mol��1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)��2O2(g)===CO2(g)��2H2O(g)����H����890.3 kJ��mol��1
B��500 �桢30 MPa�£���0.5 mol N2��1.5 mol H2�����ܱ������г�ַ�Ӧ����NH3(g)������19.3 kJ�����Ȼ�ѧ����ʽΪN2(g)��3H2(g)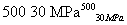 2NH3(g)����H����38.6 kJ��mol��1
2NH3(g)����H����38.6 kJ��mol��1
C����֪��120 �桢101 kPa�£�1 g H2ȼ������ˮ�����ų�121 kJ���������Ȼ�ѧ����ʽΪH2(g)��1/2O2(g)===H2O(g)����H����242 kJ��mol��1
D��25 �桢101 kPaʱ��ǿ����ǿ���ϡ��Һ������Ӧ���к���Ϊ57.3 kJ��mol��1������ϡ��Һ����������ϡ��Һ��Ӧ���Ȼ�ѧ����ʽΪ��H2SO4(aq)��2KOH(aq)�� K2SO4(aq)��2H2O(l)����H����114.6 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ��
A��Na2O2����ˮ�Ʊ�O2��2O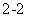 ��2H2O=4OH����O2��
��2H2O=4OH����O2��
B����ϡ����ϴ���Թ��ڱڵ�������Ag+2H��+NO =Ag��+NO��+H2O
=Ag��+NO��+H2O
C����Ba(OH)2��Һ�еμ�NaHSO4��Һ�����ԣ�Ba2����2OH����2H����SO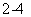 ��BaSO4����2H2O
��BaSO4����2H2O
D�� ������Һ��ͨ������CO2��2C6H5O- + CO2 + H2O  2C6H5OH + CO
2C6H5OH + CO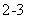
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ����������ƺϳɿ�������I�߷��ӻ�����G·�����£�
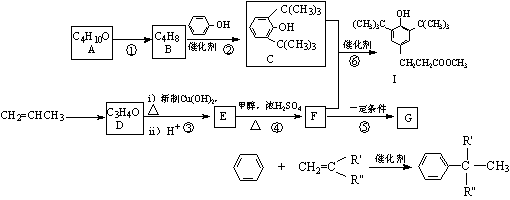 |
��֪����A�����к���3���� ��
��ش��������⣺
��1��C�ķ���ʽΪ �� A������Ϊ ��
��2����Ӧ�ٵķ�Ӧ����Ϊ ��G�Ľṹ��ʽΪ
��3��д��D������Cu(OH)2��Һ��Ӧ�Ļ�ѧ����ʽ��
��4��������Ӧ������ȡ����Ӧ���� ������ţ�
��5��F���ڶ���ͬ���칹�壬д��������������������ͬ���칹��
���ܷ���ˮ�ⷴӦ������ʹ������Ȼ�̼��Һ��ɫ �ں˴Ź���������ʾ��3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NA��ʾ����٤������������������ȷ����(����)
A��1 mol FeI2������������Ӧʱת�Ƶĵ�����Ϊ2NA
B��2 L 0.5 mol��L��1�������Һ�����������������ΪNA
C��1 mol Na2O2�����к���������Ϊ4NA
D����ϩ�ͻ�������ɵ�42 g�����������ԭ�ӵĸ���Ϊ6NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijpH=1��X��Һ�п��ܺ���Fe2+��A13+��NH4+��CO32�D��SO32�D��SO42�D��C1�D�е������֣���ȡX��Һ��������ʵ�飬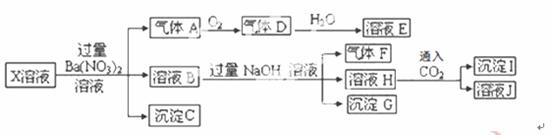 ʵ����̼��������£�
ʵ����̼��������£�
����˵����ȷ���ǣ� ��
A������A��NO2 B��X�п϶�����Fe2+��A13+��NH4+��SO42�D
C����ҺE������F���ܷ�����ѧ��Ӧ D��X�в���ȷ���������� A13+��C1�D
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com