
ij��ѧС����ʵ��������CaSO4��NH3��CO2�Ʊ�(NH4)2SO4���乤���������¡�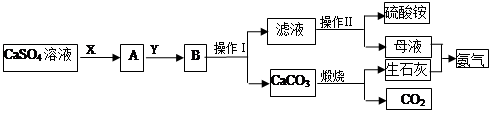
�ش��������⣺
��1�������������Ϊ_________��������һϵ�в�����������Ũ����________�����ˡ�
��2��ʵ����������̼���ʱ��ʢ��̼������õ�������________(������)��
��3��X����Ϊ____(�ѧʽ����ͬ)��Y����Ϊ____����ѭ�����õ����ʵ���_____��
��4��Ҫ�ⶨ���Ƶõ�����林��ȣ�ȡ10.0g��Ʒ����ȫ����ˮ������Һ�еμӹ������Ȼ�����Һ�����ˡ�ϴ�ӡ������������������Ϊ16.31g��Ϊ���������������Ȼ�����Һ�Ƿ������õ��Լ���_______�����Ƶ�����淋Ĵ���Ϊ________��
��5������װ�ò�������ʵ�����ư�������__________(�����)��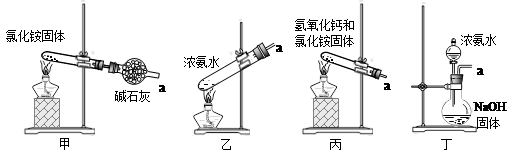
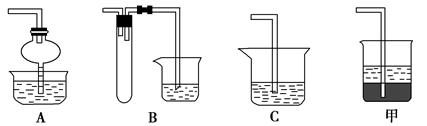
ѡ��������ȡװ�ú���������װ���ռ�����İ���������ȡ�������Һ�����ӵ�˳��(�ýӿ������ĸ��ʾ)�ǣ�a��____��____��____��____��_____��____��_____��
����װ����CCl4��������___________________��
(1)����(1��)����ȴ�ᾧ(2�֣���һ�����1��)
(2)����(1��)
(3)NH3��CO2�� NH3��CO2(ÿ�����ʾ�Ϊ1�֣����һ����һ�ֿ�1�֣���һ�ֵ���1��)
(4)�Ȼ�����Һ(2�֣�)��92.4%(3��)
(5)��(2��)��de��gf��cb��h(2��)��������(1��)
���������������1����������I�õ�CaCO3�������Һ�����Բ���IΪ���ˣ�����淋��ܽ�����¶ȵĽ��Ͷ���С����������Ũ���������ȴ�ᾧ��
��2��ʵ�����и������չ��������Ϊ������
��3��������Ŀ��Ϣ��ij��ѧС����ʵ��������CaSO4��NH3��CO2�Ʊ�(NH4)2SO4������CO2��ˮ���ܽ�Ƚ�С����NH3������ˮ��Ӧ��ͨ������NH3��ʹ��Һ�ʼ��ԣ�Ȼ����ͨ������CO2������XΪNH3��YΪCO2�����ݷ�Ӧ����ͼ���Կ����ں�����Ӧ����������CO2��NH3�����Կ�ѭ�����õ����ʵ���CO��NH3��
��4����õ��Լ����Ȼ�����Һ����Ϊ�ټ����Ȼ�����Һ�����BaSO4��������������������ᱵ�Ķ�Ӧ��ϵΪ��(NH4)2SO4 ~ BaSO4��������淋Ĵ���=16.31g��132/233��10.0g��100%=92.4%
��5���ס�ֱ�Ӽ���NH4Cl���� NH3��HCl�����Թܿڸ���NH3��HCl��Ӧ��������NH4Cl�����Բ���������ȡNH3�������ҡ�����Ũ��ˮ�ٽ�NH3�Ļӷ�������ȡNH3����ȷ�����������������ƺ��Ȼ�粒��壬�������ֽⷴӦ����NH3����������ȡNH3����ȷ������Ũ��ˮ�μӵ�NaOH�����У��ɻӷ���NH3����������ȡNH3����ȷ����ΪҪ�ռ������NH3������a���de����ʯ�Ҹ���NH3����������gf���ռ�NH3����������cb��ͨ��ϡ���ᣬ��ȡ����泥���������h������β����������NH3ֱ��ͨ��ϡ�����лᷢ����������NH3������CCl4������CCl4�������Ƿ�������
���㣺���⿼���������������������ʵ�����̵ķ����뷽����ơ����ȵļ��㡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ˮ��һ����Ҫ����Ȼ��Դ���������������治��ȱ�ٵ����ʡ�ˮ������ֱ��Ӱ�����彡������ش��������⣺
��1����Ȼˮ���ܽ��������Ҫ�� �� ���ѧʽ����
��2����������ˮ�Ĵ���ʱ��������еķ����Dzⶨˮ�� ��
��3��ˮ�ľ����������������ǣ�ˮ�ľ������û��������������ȣ�ʹ _����ˮ��������________��
��4��ͨ��ʩ��һ��ѹ��ʹˮ��������Ĥ��������ӻ����ӽ������Ӷ�ʹˮ���Ծ����ķ�����Ϊ ��������������ˮʱ��ʹ����ͨ����Ĥ���ƶ����� ��
��5��ij��Ȼˮ�� ��
��
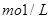 ��Ҫ����10 m3������Ȼˮ�����ȼ���Ca��OH��2 g���ټ���Na2CO3 g��
��Ҫ����10 m3������Ȼˮ�����ȼ���Ca��OH��2 g���ټ���Na2CO3 g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��18�֣��������������գ���Ŀǰ����ˮ���塱������Ҫ����֮һ���乤���������£�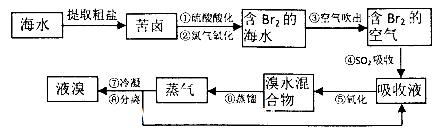
��1����Ԫ�������ڱ���λ��_______ ����_______�塣
��2����������������ữ�����Cl2�������ʣ�������________ ��
��3�������������SO2�Ļ�ԭ�ԣ���Ӧ�����ӷ���ʽΪ_________ __��
��4���������������У��¶�Ӧ������80��90���¶ȹ�����Ͷ������������������ԭ��____________________________.
��5���������������������õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��롣����������������__________��
��6������١���֮��δֱ���á���Br2�ĺ�ˮ����������õ�Һ�壬���Ǿ�������������������SO2���ա�����������������������������������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
4,7-�����㶹�أ��۵㣺132.6�棩��һ����Ҫ�����ϣ��㷺�ֲ���ֲ�����,�ɼ�ױ���Ϊԭ�ϵĺϳɷ�Ӧ���£�
ʵ��װ��ͼ���£�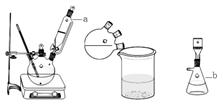
��Ҫʵ�鲽�裺
����1.��������ƿ�м���60mLŨ���ᣬ����ȴ��0�����£������µ����ױ���30mL(0.29mol)��������������26.4mL (0.21mol)�Ļ���
����2.������10���£�����12h����Ӧ��ȫ���䵹���ˮ������У�Ȼ����ˡ�ˮϴ�ô�Ʒ
����3.��Ʒ���Ҵ��ܽⲢ�ؽᾧ���ð�ɫ��״���岢��ɣ��Ƶò�Ʒ����Ϊ33.0g��
��1��ͼ����Ʒ���ƣ�a ��b ��
��2��ŨH2SO4��Ҫ��ȴ��0�����µ�ԭ���� ��
��3����Ӧ��Ҫ����12h����ԭ���� ��
��4��ȷ�����ղ�Ʒ��4,7-�����㶹�ص�ʵ����� ��
��5������ʵ�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��˾ƥ�֣�����ˮ���ᣬ ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135�档ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135�档ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�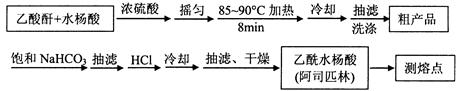
��Ҫ�Լ��Ͳ�Ʒ����������
�����������Ϣ�ش��������⣺
��1���ٺϳɰ�˹ƥ��ʱ������ʵļ��ȷ�ʽ�� ��
�ڳ������ôֲ�ƷҪ��������ˮϴ�ӣ���ϴ�ӵľ�������� ��
��2���ᴿ�ֲ����м��뱥��NaHCO3��Һ��û��CO2����Ϊֹ���ٳ��ˣ���ӱ���NaHCO3��Һ��Ŀ���� ��
��һ�ָĽ����ᴿ��������Ϊ�ؽᾧ�ᴿ�����������£�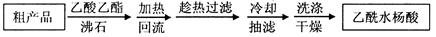
��3���Ľ����ᴿ�����м��Ȼ�����װ����ͼ��ʾ��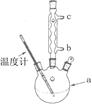
��ʹ���¶ȼƵ�Ŀ���� ������ˮ������������ �����b����c������
���ؽᾧ�ᴿ�����ò�Ʒ���л�����Ҫ��ԭ�����ٵ�ԭ�� ��
��4�������Ʒ���Ƿ���ˮ����Ļ�ѧ������ ��
��5����ѧϰС����ʵ����ԭ��������2.0 gˮ���ᡢ5.0 mL���������ѣ�1.08 g/cm3)�����ճƵò�Ʒm��2.2 g������������ˮ����IJ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ��̽��ʳƷ���Ӽ������NH4Al(SO4)2��12H2O���·ֽ�������
��1��Ԥ�������й�����������Ԥ�ⲻ�������� ��
A��NH3��N2��SO2��H2O B��NH3��SO3��H2O
C��NH3��SO2��H2O D��NH3��N2��SO3��SO2��H2O
��2�����Լ��飺ȡһ������������������ʵ��̽�����
�ٰ�ͼʾ��װ���������ȼ������װ�õ������ԣ������� ��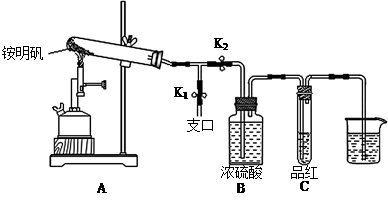
�ڼ�סֹˮ��K1����ֹˮ��K2���þƾ���Ƴ�����ա�ʵ������У�װ��A�͵�����δ������ɫ���壻�Թ�C�е�Ʒ����Һ��ɫ����֧�ڴ��ɼ��鵽NH3�������� ����װ��A��B֮���T�͵����г��ְ�ɫ���壬�ð�ɫ��������� ������һ�����ʵĻ�ѧʽ����
�۷����ó�װ��A�Թ��в����İ�ɫ���������������д��������NaOH��Һ�����ӷ���ʽ ��
��Ϊ�˷�ֹ������ʵ�����ʱ������ ������ĸ��ţ���Ȼ��Ϩ��ƾ���ơ�
A��ȡ���ձ��еĵ��� B����ֹˮ��K1 C���ر�ֹˮ��K2
��3�������ͽ��ۣ�ʵ��֤����������ǣ�1��D�е�5�����塣��ͬ�����²������N2��SO2��������Ƕ�ֵ��V��N2����V��SO2��= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�屽��һ�ֻ���ԭ�ϣ�ʵ���Һϳ��屽��װ��ʾ��ͼ���й��������£�
| | �� | �� | �屽 |
| �ܶ�/(g��cm��3) | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ˮ���ܽ�� | �� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������C9H10O2������Ϊ��Ϣ��������������һ����ɫ��Һ�壬������ˮ������ˮ����ζ������������ˮ�㾫�����쾫�ͣ���������ʳƷ��ҵ�У�Ҳ�������л��ϳ��м��壬�ܼ��ȡ����Ʊ�����Ϊ��
��֪��
| ���� | ��Է������� | ��ɫ��״̬ | �е�(��) | �ܶ�(g��cm-3) |
| ������* | 122 | ��ɫƬ״���� | 249 | 1.2659 |
| ���������� | 150 | ��ɫ����Һ�� | 212.6 | 1.05 |
| �Ҵ� | 46 | ��ɫ����Һ�� | 78.3 | 0.7893 |
| ������ | 84 | ��ɫ����Һ�� | 80.8 | 0.7318 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�������д�����CuS���������������P��������������������ʡ�ij��ѧ����С������������̣��Ըÿ���Ϊԭ������CuCl2��2H2O���塣
��֪����20 ��ʱ���Ȼ�ͭ���ܽ����73 g�������£��������ӿ�ʼ�����ͳ�����ȫʱ��pH���±���
| �������� | ��ʼ�γ��������������pH | ��ȫ�γ��������������pH |
| Fe2�� | 7.0 | 9.0 |
| Fe3�� | 1.9 | 3.2 |
| Cu2�� | 4.7 | 6.7 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com