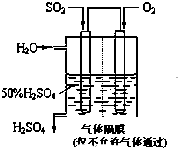
���ô�������Ӧ��SO
2ת��ΪSO
3�ǹ�ҵ����������Ĺؼ����裮��֪��
SO
2��g��+
O
2��g��?SO
3��g����H=-98kJ?mol
-1��
��1��ij�¶��¸÷�Ӧ��ƽ�ⳣ��K=
�����ڴ��¶��£���100L�ĺ����ܱ������У�����3.0mol SO
2��g����16.0mol O
2��g����3.0mol SO
3��g������Ӧ��ʼʱv������
v���棩�����������������=������
��2��һ���¶��£���һ�����������Ϊ2L���ܱ������г���2.0mol SO
2��1.0mol O
2���ﵽƽ��������Ϊ1.6L����SO
2��ƽ��ת����Ϊ
��
��3���ڣ�2���еķ�Ӧ�ﵽƽ��ı�������������ʹSO
2��g��ƽ��Ũ�ȱ�ԭ����С����
������ĸ����
A�������¶Ⱥ�����������䣬����1.0mol O
2B�������¶Ⱥ�������ѹǿ���䣬����1.0mol SO
3C�������¶�
D���ƶ�����ѹ������
��4������ͼ��ʾװ�ã��õ绯ѧԭ���������ᣬд��ͨ��O
2�缫�ĵ缫��ӦʽΪ
��Ϊ�ȶ�����������������Һ��Ũ��Ӧά�ֲ��䣬��ͨ��SO
2��ˮ��������Ϊ
��

 ���ô�������Ӧ��SO2ת��ΪSO3�ǹ�ҵ����������Ĺؼ����裮��֪��
���ô�������Ӧ��SO2ת��ΪSO3�ǹ�ҵ����������Ĺؼ����裮��֪��


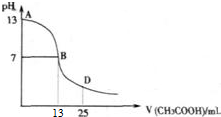
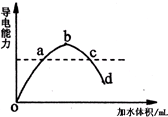 ��һ���¶��£��������ˮϡ�����У���Һ�ĵ���������ͼ��ʾ����ش�
��һ���¶��£��������ˮϡ�����У���Һ�ĵ���������ͼ��ʾ����ش�