
(11��)ij��ѧС�����ʵ��̽��̼���ơ�̼�����Ƶ����ʣ�ʵ�����£�ȡ��֧�Թֱܷ����10 mL��ͬŨ�ȵ�ϡ���ᣬ��������װ��0 5 g��Na2CO3��NaHCO3��ĩ��С����ֱ����������Թ��ϣ��������ڵĹ����ĩͬʱ�����Թ��У���֪�����������۲�ʵ������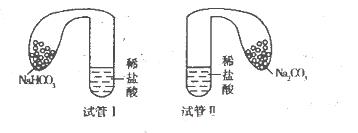
(l)��֧�Թ��о��������壬���в�������Ͽ��Ϊ________(��Թ�I�����Թ�II��)��Ͷ��________���ѧʽ�����Թ��������ñȽϴ�
��2����ͬѧ�����������Թܣ������Թ�I���䣬�Թܢ���ȣ��ɴ����������״̬��Σ�NaHCO3��HCl��ӦΪ���ȷ�Ӧ��Na2CO3��HCl��ӦΪ���ȷ�Ӧ��
Ϊ��һ��̽��Na2CO3��NaHCO3�����ᷴӦ�������仯����ͬѧ��������ʵ�飬��������Ϊ�������Լ�1�м����Լ�2�����衢�ⶨ�¶ȣ��ھ��á��ⶨ�¶ȣ����ټ����Լ�3�����衢�ⶨ�¶ȡ���¼���õ��������ݣ�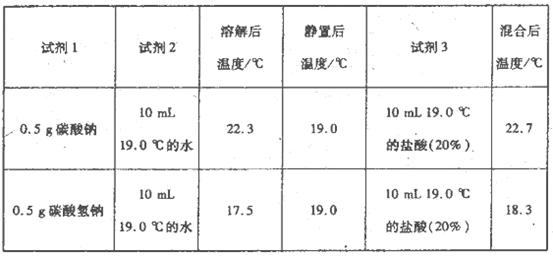
����ʵ����Ҫ�õ��IJ���������________��
��ͬѧ�ɵó����ۣ�
��NaHCO3���ܽ����________������ȡ����ȡ�����ͬ����Na2CO3���ܽ����________��
��CO32����H����ӦΪһ��Ӧ������ȡ������ȡ�����ͬ����HCO3����H����ӦΪ________��Ӧ��
��3���Ƚϼ���ͬѧ��ʵ�飬����Ϊ ________����ס����ҡ�����ʵ�鷽���������������ܡ�
��1���Թ�I��NaHCO3��
��2���ձ������������¶ȼƣ������ȡ����ȣ��ڷ��ȡ����ȣ�
��3����
���������������1��̼��������̼�������Ũ�ȵ����ᷴӦ��̼�����Ƶķ�Ӧ���ʿ죬��Ϊ̼������ֻ����1�������ӾͿ����ɶ�����̼���壬���Բ�������Ͽ�����Թ�I����������̼��������̼�������������ᷴӦ��̼�����Ʋ���������࣬����Ͷ��NaHCO3���Թ��������ñȽϴ�
��2������ʵ������жϣ���Ҫ�IJ����������ձ������������¶ȼƣ�
�ٴӱ������ݿ�֪��ʱ������19��0ºC��̼�������ܽ���¶ȵ���19��0 ºC��˵��̼�����Ƶ��ܽ�������ȣ�ͬ��̼���Ƶ��ܽ�����Ƿ��ȣ�
��̼����������ķ�Ӧ��2����CO32��+H��=HCO3����HCO3��+ H��=H2O+CO2������̼�����Ƶķ�Ӧ�¶��ж�HCO3����H����ӦΪ���ȷ�Ӧ����̼����������ķ�Ӧ�Ƿ��ȷ�Ӧ������̼���������ᷴӦ�ĵ�һ���Ƿ��ȷ�Ӧ��
��3����ͬѧ��ʵ�鷽������������ͬѧ���жϿ�������Ϊ�ܽ���̵���ЧӦ��ɵ��¶ȵIJ��죬����˵���Ƿ�Ӧ����ЧӦ��ɵģ���ͬѧͨ���ȽϷ�Ӧ����¶ȣ��жϷ�Ӧ����ЧӦ�������ȸ���˵������
���㣺����̼�����ơ�̼���Ƶ�����ʵ�飬��ʵ��ķ����ж�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ʵ���������ʵ��Ԥ��ʵ��Ŀ�Ļ����ý���һ�µ���( )
| ѡ�� | ʵ���������ʵ | ʵ��Ŀ�Ļ���� |
| A | ij��Һ ð������ ð������ | ˵��ԭ��Һ��һ������CO32- |
| B | SiO2�봿����¿�����CO2 | ˵����������Ա�̼��ǿ |
| C | �⻯����Һ�����Ի�ɫ | ������I-����ԭ��������I2������Һ�� |
| D | ��������Ũ�����н��ݺ���������ˮ��ϴ��Ȼ�����CuSO4��Һ�в���Ӧ | ˵�����������γ���һ�������ȶ�������Ĥ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����п��ĩ�㷺Ӧ������Ϳ�ϡ��մɡ�������ҽҩ����������ҵ��Ϊ�ۺ�Ӧ����Դ������ұ��п��п��Ʒ�ӹ���ҵ���յ�п��������ZnO��FeO��Fe2O3��CuO��Al2O3�����ʣ�����ȡ����п��������ͼ��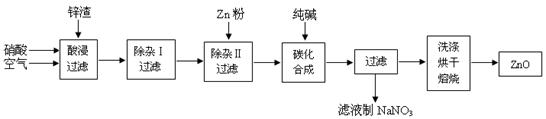
�й��������������ȫ��pH���±���
��1������������У�����п����ɷ�ĩ��ͨ�������ͬʱ����������������� ��
��2���������նദ�漰�����ˡ���ʵ�����й�����Ҫʹ�õIJ����������ձ��� ��
��3�����ڡ����Ӣ��У���������KMnO4��Һ����Ŀ���� ��KMnO4�Ǹ÷�Ӧ�� ������������ԭ����������Һ��pH����4��Ŀ���� ��
���ڡ�����II���У�����п�۵�Ŀ���� ��
��4���ڡ�̼���ϳɡ��У��������м�ʽ̼��п��Zn2(OH)2CO3�ݺ�CO2�ȣ���д���÷�Ӧ�Ļ�ѧ����ʽ ��
��5���������У����˷�������϶࣬�����Ե�ȱ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ�к��е���Ҫ��������������������ij��ѧ��ȤС���ô���ʯΪԭ����ȡ��ȫ��ɱ�����������Ƶ���Ҫ���̣�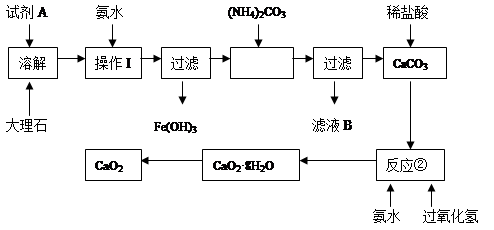
��ش��������⣺
��1���Լ�A�������� ��
��2������I��Ŀ���� ��
��3����ʵ����Ҫ���ʹ�ù��ˣ������С�һ���������͡��������������С����͡�ָ ��
��4��д����Ӧ��������CaO2��8H2O�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����16�֣���ѧʵ���ǿ�ѧ̽���Ļ�������ش��й�ʵ�����⣺
��1��������ĸ�ʵ��װ������������������ȱ�ݣ�������ȫ��ȷ���� ��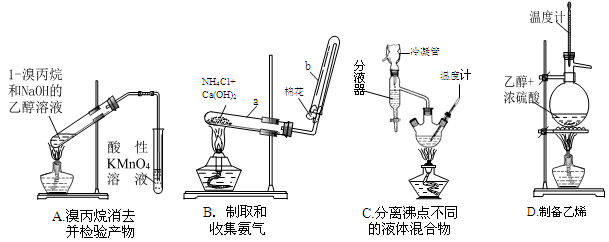
��2��Ҫ��������Bװ�ð����Ѽ����IJ����� ���Թ��Ѽ�����
��3��ClO2��һ�ְ�ȫ����Ч�����ס�ǿ��ɱ���������������ұ���
�����±����Կ���������Һ̬ClO2���������ܷ⡢ ��ClO2��Ӧ�����ӷ���ʽ ���۲��¡�ͼA����Ҫ��NaClO2��Һ�Ƶò����ᾧˮ�ľ��壬�����������ᾧ������������ ��Ӧ��������������¶ȷ�Χ�� ��
| ɫ̬ | ���ڼ� | ����1Kpa�����Ȼ����� | |
| �������� | -59-11�� ���ɫҺ�� | �����������κ������� | ��ը |
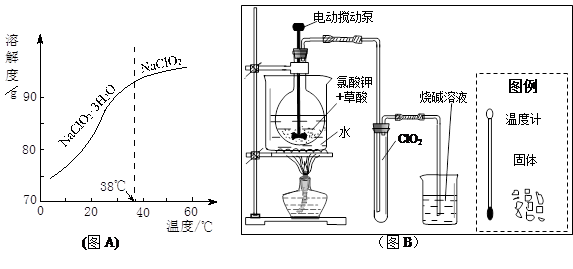
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��14�֣�ˮ�����л�������нϸߵĻ�ѧ����������������KMnO4�����л�����京������Ҫ�����������£�
��1������AΪ ������ʽ���ʽ���ζ��ܣ�Ҫ˳�����ʵ�飬���������Һ��ɫӦΪ ��
��2�����Ʋ�����漰�����ӷ���ʽ��
C2O42��+ MnO4��+ = Mn2++ CO2��+
�ò����е����һ��Na2C2O4ʱ��ɫ�������Ժ�ĵζ�����ɫ�Ͽ죬��ԭ���� ��
��3���������������Na2C2O4��ҺΪ20.00ml����֪�ζ����Һ����ͼ��ʾ������ͼ�б���ζ�ǰ��Һ�档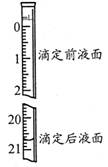
��4����ʵ����������ʵ���л��ﺬ��ƫ�ߣ��ֱ����������������룺
����1��ˮ����Cl��Ӱ��
����2������Na2C2O4��Һ����ʱ ��
��5��������1��������������Ͽ�Ƭ������������ʵ��������Cl��Ӱ�졣����ѡ�Լ���AgNO3��Һ��Ag2SO4��Һ��KMnO4��Һ��Na2C2O4��Һ�� ��
���Ͽ�Ƭ��
1���л��ʿ�HNO3��������
2��AgCl��������KMnO4��Һ��Ӧ��
3��Ag2C2O4�ɱ�����KMnO4��Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��15�֣�S2Cl2�����������л����Ȼ����Լ���ʵ���ҿ���������װ�����Ʊ�S2Cl2�������ּг���������ȥ��
��֪�����Ʊ��ķ�Ӧ����ʽΪ��
�ڷ�Ӧ�漰�ļ��������������£�
| ���� ���� | �۵� | �е� | �Ż�� | �������� |
| ��� | 119.2�� | 446.6�� | 363�� | / |
| ��� | 112.8�� | 446.6�� | 363�� | / |
| S2Cl2 | -77�� | 137�� | / | ��ˮǿ�ҷֽ�����S��SO2��HCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����г����⣬����������Ԫ�أ���̼Ԫ�غ���Ԫ�ء�����̼��Ҫ��̼��������̬���ڣ���ʹ�������ܼ�Ӳ���࣬������������;����һ���������ֵ�ԭ�ϡ�ij��ȤС����ư���ͼ��ʾ��ʵ��װ�ã��ⶨ�����еĺ�̼����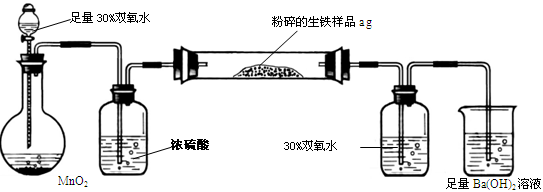
A B C D E
��ش��������⣺
��1���������������к�Ԫ�أ���ʹ���������ȴ��ԡ���Ԫ�������������п��ܴ��ڵļ�̬��
| A����2���� | B��0�� �� | C��+4���� | D��+6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ�ⶨ��������茶��塾��NH4��2Fe (SO4)2 �� xH2O�������ĺ�����ijʵ��С����������ʵ�飺
����һ���õ�����ƽȷ����5.000g��������茶��壬���Ƴ�250ml��Һ��
�������ȡ������Һ25.00ml����ƿ�У���ϡH2SO4�ữ����0.010mol/L KMnO4��Һ�ζ���Fe2+ǡ��ȫ��������Fe3+��ͬʱ��MnO4-����ԭ��Mn2+��
���ظ���������Ρ�
��ش��������⣺
��1�����������������Һ�IJ������������ǣ������� ��ת�ơ�ϴ�Ӳ�ת�ơ� ��ҡ�ȡ�
��2���� �ζ���ʢ��KMnO4��Һ��
��3�����������һ��KMnO4��Һ,���� ,������ζ��յ㡣��Ӧ�����ӷ���ʽ��
��4���ζ�������±���ʾ��
| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.05 | 21.04 |
| 2 | 25.00 | 1.50 | 24.50 |
| 3 | 25.00 | 0.20 | 20.21 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com