��������1������c=
�������Ũ�����Ũ�ȣ�Ȼ�����ϡ���������ʵ����ʵ�������������ҪŨ����������
��2�����ݷ�Ӧ��֪��������������ʵ�����������������ӵ����ʵ�����ȣ��ݴ˼������Ӧ����Һ�����������Ũ�ȣ�
��3�����ݼ��������ж����л����ǡ����ȫ��Ӧ��Ȼ������������Cu
2S��CuS�����ʵ������ֱ������������������������ʵ�����ʽ���㣻
��4����ϣ�3���������Ӧ�����������ӵ����ʵ������ٸ���c=
���������������Ũ�ȣ��������ʣ��������Ũ�ȣ�
��5�����ݣ�3�������64.0g������к��е�CuS��Cu
2S�����ʵ�����Ȼ�����ü�ֵ���ֱ���������NO�����ʵ�����������Ӷ��ó�V�ķ�Χ��
���
�⣺��1����������Ϊ0.63���ܶ�Ϊ1.42g/cm
3��Ũ�����Ũ��Ϊ��
=14.2mol/L������100mL��5mol/L��ϡ���ᣬ���ƹ�������������ʵ������䣬����Ҫ��Ũ��������Ϊ��
��0.0352L=35.2mL��
�ʴ�Ϊ��35.2mL��
��2�����ݷ�Ӧ��3CuS+8H
++8NO
3-��3Cu
2++3SO
42-+8NO��+4H
2O����3Cu
2S+16H
++10NO
3-��6Cu
2++3SO
42-+10NO��+8H
2O��֪����Ӧ����NO��������ʵ�����������������ӵ����ʵ�����ȣ���ʵ��������NO�����ʵ���Ϊ��
=0.225mol�����������Ũ��Ϊ��
=2.25mol/L����Ӧ����ҺŨ��Ϊ��5mol/L-2.25mol/L=2.75mol/L��
�ʴ�Ϊ��2.75mol/L��
��3��9.6g���������5.04LNO���壬12.8g������ܹ�����һ�����������Ϊ��
��5.04L=6.72L��˵�����л������ȫ��Ӧ��
��12.8g����Ʒ��CuS�����ʵ���Ϊx��Cu
2S�����ʵ���Ϊy��
���������ɵã���96x+160y=12.8��
������������ɵã���
x+
y=
=0.3��
���ݢ٢ڽ�ã�x=y=0.05mol��
���Ի������Ʒ��Cu
2S��CuS�����ʵ���֮��Ϊ��1��1��
�𣺻������Ʒ��Cu
2S��CuS�����ʵ���֮��1��1��
��4�����ݷ���ʽ�١��ڿ�֪���������ӵ����ʵ���Ϊ��n��H
+��=0.05mol��
+0.05mol��
=0.4mol��
�����������ӵ�Ũ��Ϊ��c��H
+��=
=4mol/L��
����ʣ��������Ũ��Ϊ��c��H
+��=5mol/L-4mol/L=1mol/L��
����ʵ���������Һ�������ӵ�Ũ��Ϊ1mol/L��
��5��100mL 5mol/L��ϡ�����к�����������ʵ���Ϊ��5mol/L��0.1L=0.5mol��
���ݣ�3����֪��64g��Ʒ��CuS��Cu
2S�����ʵ���Ϊ��n��Cu
2S��=n��CuS��=0.05mol��
=0.25mol��
��HNO
3ֻ��CuS��Ӧ��3CuS+8H
++8NO
3-��3Cu
2++3SO
42-+8NO��+4H
2O��0.25molCuS��ȫ��Ӧ�������0.25mol��
��0.5mol������㣬����ݷ�Ӧ��֪����NO�����ʵ���Ϊ��0.5mol�������NO���Ϊ��V��NO��=0.5mol��22.4L/mol=11.2L��
��HNO
3ֻ��Cu
2S��Ӧ��3Cu
2S+16H
++10NO
3-��6Cu
2++3SO
42-+10NO��+8H
2O��0.25molCu
2S�������0.25mol��
��0.5mol������㣬���ݷ�Ӧ��֪����NO�����ʵ���Ϊ��0.5mol��
=
mol�������NO�����22.4L/mol��
mol=7L��
���Ա��в������������V��Ϊ��7L��V��11.2L��
�𣺱�ʵ���в�����������Ϊ��7L��V��11.2L��




 ������ķ��㰷����¶�ڿ����б�Ϊ��ɫ���ñ仯���п����뱽������
������ķ��㰷����¶�ڿ����б�Ϊ��ɫ���ñ仯���п����뱽������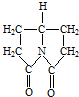 д�����л����������ˮ�����Ľṹ��ʽ
д�����л����������ˮ�����Ľṹ��ʽ
 25��ʱ����ʢ��50mL pH=2��HA��Һ�ľ��������м���pH=13��NaOH��Һ������NaOH��Һ�������V�������û����Һ���¶ȣ�T���Ĺ�ϵ��ͼ��ʾ������������ȷ���ǣ�������
25��ʱ����ʢ��50mL pH=2��HA��Һ�ľ��������м���pH=13��NaOH��Һ������NaOH��Һ�������V�������û����Һ���¶ȣ�T���Ĺ�ϵ��ͼ��ʾ������������ȷ���ǣ������� ��װ����ȡ����
��װ����ȡ���� ��װ�÷���������̺��Ȼ�����Һ
��װ�÷���������̺��Ȼ�����Һ ��װ�÷�����ȡ���ˮ�е�
��װ�÷�����ȡ���ˮ�е�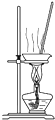 ��װ�������Ȼ�����Һ�� MnCl2?4H2O
��װ�������Ȼ�����Һ�� MnCl2?4H2O