
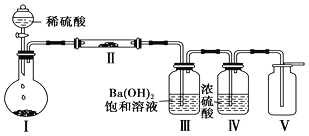
����Ŀ����ͼ����ʵ���ҽ��ж��������Ʊ�������ʵ������װ�ã����̶ֹ�װ��δ������
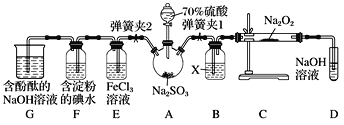
(1)����װ��װ�ú���Ҫ����A��Dװ�õ������ԣ������������__________��Ȼ����D��װ��ˮ��Ȼ����A���۲쵽D��������ð�����ƿ��ƾ��ƻ��ɿ�˫�֣�D�е�����ˮ���γ��Ҹ߶ȱ��ֲ��䣬˵��װ�����������á�
(2)װ��D��ʢ��NaOH��Һ��������___________��
(3)�رյ��ɼ�1���ɼ�2�������������E��F��G�У���˵��I����ԭ������SO2������Ϊ_____��������Ӧ�����ӷ���ʽ��________________��
(4)Ϊ����֤E��SO2��FeCl3������������ԭ��Ӧ�����������ʵ�飺ȡE�е���Һ������Һ�м�����ϡ�����ữ��BaCl2��Һ��������ɫ������˵��SO2��FeCl3������������ԭ��Ӧ��
���������Ƿ������________(����������������������)��ԭ����_____________��
(5)ʵ�������G�к���̪��NaOH��Һ����ɫ����ʵ��֤��SO2����Ư���Ի���������ˮ�����ԣ������ʵ����֤��______________________��
���𰸡��رյ��ɼ�2�ͷ�Һ©�����������ɼ�1 ����δ��Ӧ��SO2����ֹ��Ⱦ���� F����Һ��ɫ��ȥ SO2��I2��2H2O===2I����SO42-��4H�� ������ E���ܽ��SO2��ϡ���ᷴӦҲ����SO42- ȡ��ɫ�����Һ���μ�NaOH��Һ������죬��֤����ɫ����SO2����ˮ������(��ȡ��ɫ�����Һ�����ȣ���δ���ɫ����֤��SO2����ˮ������)
��������
��1������װ�õ�������ʱ����Ҫ�γ��ܷ�ϵͳ��
��2��SO2���ж�������������ܱ�NaOH��Һ���ա�
��3��I����ԭ������SO2������I2��SO2�ķ�Ӧ��ɵó�ʵ����������ӷ���ʽ��
��4������ʵ�����ʱ������Ҫ��������������ԭ��Һ�����ӵķ�Ӧ�Ƿ���ʵ����ɸ��š�
��5��ȡ��ɫ�����Һ���μ�NaOH��Һ������죬��֤����ɫ����SO2����ˮ������(��ȡ��ɫ�����Һ�����ȣ���δ���ɫ����֤��SO2����ˮ������)��
��1������װ�õ�������ʱ����Ҫ�γ��ܷ�ϵͳ�����Թرյ��ɼ�2�ͷ�Һ©�����������ɼ�1���ʴ�Ϊ���رյ��ɼ�2�ͷ�Һ©�����������ɼ�1��
��2��SO2���ж�������������ܱ�NaOH��Һ���գ��ʴ�Ϊ������δ��Ӧ��SO2����ֹ��Ⱦ������
��3����ˮ������SO2����������ԭ��Ӧ���������������ᣬ���ӷ���ʽΪ��SO2��I2��2H2O===2I����SO42-��4H������Ϊ��ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ļ�ԭ�ԣ�����I����ԭ������SO2��F�еⵥ�ʱ�ɵ������ˣ�����Һ��ɫ��ȥ���ʴ�Ϊ��F����Һ��ɫ��ȥ��SO2��I2��2H2O===2I����SO42-��4H����
��4������������Ϊϡ�������ǿ�����ԣ��ܰѶ�������������SO42-���ʴ�Ϊ����������E���ܽ��SO2��ϡ���ᷴӦҲ����SO42-��
��5��ʵ�������G�к���̪��NaOH��Һ����ɫ��ȡ��ɫ�����Һ���μ�NaOH��Һ������죬��֤����ɫ����SO2����ˮ������(��ȡ��ɫ�����Һ�����ȣ���δ���ɫ����֤��SO2����ˮ������)���ʴ�Ϊ��ȡ��ɫ�����Һ���μ�NaOH��Һ������죬��֤����ɫ����SO2����ˮ������(��ȡ��ɫ�����Һ�����ȣ���δ���ɫ����֤��SO2����ˮ������)��


 ������ϵ�д�
������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������0.1molCH3COONa��0.05molHCl����ˮ���1L��Һ(pH��7).
��1�������ӷ���ʽ��ʾ����Һ�д��ڵ�����ƽ����ϵ______________��_______________��___________________
��2����Һ�и����ӵ����ʵ���Ũ���ɴ�С˳��Ϊ_____________________________________________________
��3����Һ��������Ũ��Ϊ0.1mol/L����________________��Ũ��Ϊ0.05mol/L����____________________
��4�����ʵ���֮��Ϊ0.lmol������������______________��__________
��5��CH3COO-��OH-�����ʵ���֮�ͱ�H+��________mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ���Ӧ�������������

A. ��ͼ�����жϣ����ڷ�ӦA(g)��B(g) ![]() 2C(g)����T1>T2������H<0
2C(g)����T1>T2������H<0
B. ͼ�ұ�ʾ���淴ӦCO(g)��H2O(g) ![]() CO2(g)��H2(g)����H>0
CO2(g)��H2(g)����H>0
C. ͼ����ʾCO2ͨ�뱥��Na2CO3��Һ�У���Һ�����Ա仯
D. ͼ����ʾ0.1 mol��L��1������ζ�20 mL 0.1 mol��L��1 NaOH��Һ����ҺpH�������������ı仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijʵ��С��������װ�ý����Ҵ���������ʵ�顣

��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ����ʽ______________________________��________________________________��
��2����ˮԡ��������_________________________________________����ˮԡ��������_________________________________________��
��3����Ӧ����һ��ʱ����Թ�a�����ռ�����ͬ�����ʣ�������________������ƿ���ռ������������Ҫ�ɷ���________��
��4�����Թ�a���ռ�����Һ������ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����______��Ҫ��ȥ�����ʣ����ڻ��Һ�м���____________����д��ĸ����
�ᣮ�Ȼ�����Һ������b�������� c��̼��������Һ ������d�����Ȼ�̼
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������X��һ�����ϣ��ɲ�����ϩ��ױ�Ϊ��Ҫԭ�ϣ�������·�ߺϳɣ�
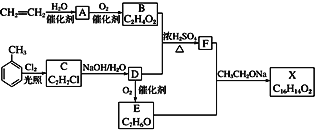
��֪��RX![]() ROH��
ROH��
RCHO��CH3COOR��![]() RCH===CHCOOR��
RCH===CHCOOR��
��ش�
(1)E�й����ŵ�������________��D��E�ķ�Ӧ����_______��
(2)B��D��F�Ļ�ѧ����ʽ_____________________C��D�Ļ�ѧ����ʽ___________________��
(3)X�Ľṹ��ʽ____________________��
(4)���ڻ�����X������˵����ȷ����________��
A���ܷ���ˮ�ⷴӦ B������Ũ���ᷢ��ȡ����Ӧ
C����ʹBr2/CCl4��Һ��ɫ D���ܷ���������Ӧ
(5)���л�����������F��ͬ���칹�����_________��
C��CH2===CHCH===CHCH===CHCH===CHCOOH
![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��W���ֻ�������ɶ�����Ԫ����ɡ�����X��������Ԫ�أ�X��Y��Z����ɫ��Ӧ��Ϊ��ɫ��WΪ��ɫ��ζ���塣�����ֻ������������ͼת����ϵ(���ַ�Ӧ����P��Ӧ��������ȥ)����ش�
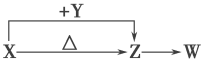
(1)Y�ĵ���ʽ��____________________��
(2)X��Y����Һ�з�Ӧ�����ӷ���ʽ��__________________________________________��
(3)X���е�����Ԫ��֮��(���֡����ֻ�����)����ɶ��ֻ����ѡ������ijЩ�����������ͼװ��(�г̶ֹ�װ������ȥ)����ʵ�飬װ�â��в�����ɫ������װ�â��п��ռ���һ����ɫ���塣
��װ�â��з�Ӧ�Ļ�ѧ����ʽ��_________________________________________________��װ�â��з�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
����X���е�����Ԫ���е�������ɵ�ij������ڴ����������Ʊ����ռ����������װ�â������壬�û�����Ļ�ѧʽ��___________����������װ����_______________�� (����ͼѡ���Ҫװ�ã���д���)��
(4)��Z��Һ��ͨ�����������Ƶ�ij�������������г��õ�Ư�ס����������ʣ�ͬʱ��X���ɣ��÷�Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����ƵĻ�����������������ǣ� ��
A.�������ƿ����ں����������Ϊ��������Դ
B.����������ˮ��Ӧ�ų�����
C.̼���ƽ�����ɫ��Ӧ������ʻ�ɫ
D.�����£��������ǰ�ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ����д�����۾���������( )
ѡ�� | ���ӷ���ʽ | ���� |
A | ��2 mol Cl2ͨ�뺬1 mol FeI2����Һ�У� 2Fe2����2I����2Cl2===2Fe3����4Cl����I2 | ��ȷ��Cl2�������ɽ�Fe2����I�������� |
B | Ba(HCO3)2��Һ��������NaOH��Һ��Ӧ�� Ba2����HCO3-��OH��===BaCO3����H2O | ��ȷ����ʽ����Ӧ�������κ�ˮ |
C | ����SO2ͨ��NaClO��Һ�У� SO2��H2O��ClO��===HClO��HSO3- | ��ȷ��˵�����ԣ�H2SO3ǿ��HClO |
D | 1 mol/L��NaAlO2��Һ��2.5 mol/L��HCl��Һ�������ϣ� 2AlO2-��5H��===Al3����Al(OH)3����H2O | ��ȷ����һ����Ӧ�͵ڶ�����Ӧ���ĵ�H�������ʵ���֮��Ϊ2��3 |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com