
����Ŀ����֪����A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ������Ժ������ҵ�ʯ�ͻ���ˮƽ����2CH3CHO��O2![]() 2CH3COOH ����֪����E�Ľṹ��ʽΪ
2CH3COOH ����֪����E�Ľṹ��ʽΪ![]() ������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��
������AΪ��Ҫԭ�Ϻϳ�������������ϳ�·������ͼ��ʾ��
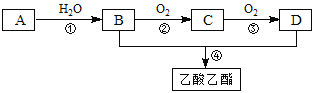
�ش��������⣺
(1)AΪ______________(��ṹ��ʽ) CΪ________________(������)
(2)E�����������еĹ�������_______________��________________(������)
(3)д���ڢںܲ͢���Ӧ�Ļ�ѧ����ʽ��
�ڵķ���ʽ______________________________________________����Ӧ����_________
�ܵķ���ʽ______________________________________________����Ӧ����__________
(4)д��E�������Ʒ�Ӧ�ķ���ʽ__________________________________________��
(5)д������E������һ�������·�Ӧ������Ԫ��״���Ļ�ѧ����ʽ________________��
���𰸡�CH2��CH2 ��ȩ �ǻ� �Ȼ� 2CH3CH2OH+O2![]() 2CH3CHO+2H2O ������Ӧ CH3COOH��CH3CH2OH
2CH3CHO+2H2O ������Ӧ CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O ȡ����Ӧ��������Ӧ
CH3COOCH2CH3��H2O ȡ����Ӧ��������Ӧ ![]() ��2Na��H2����
��2Na��H2����![]() 2
2![]()
![]() 2H2O��
2H2O��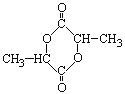
��������
A��ʯ���ѽ�������Ҫ�ɷݣ�A�IJ���ͨ����������һ�����ҵ�ʯ�ͻ���ˮƽ����AΪCH2=CH2����ˮ�����ӳɷ�Ӧ����BΪCH3CH2OH���Ҵ���������CΪCH3CHO��CH3CHO��һ�������ɵ�DΪCH3COOH��CH3COOH��CH3CH2OH����������Ӧ����CH3COOCH2CH3���Դ������
(1)���ݷ����ɵã�AΪCH2=CH2��CΪ��ȩ��
(2)E�Ľṹ��ʽΪ��![]() ��E�����������еĹ��������ǻ����Ȼ���
��E�����������еĹ��������ǻ����Ȼ���
(3)�ڵķ���ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O����Ӧ����Ϊ������Ӧ���ܵķ���ʽΪCH3COOH��CH3CH2OH
2CH3CHO+2H2O����Ӧ����Ϊ������Ӧ���ܵķ���ʽΪCH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O����Ӧ����Ϊ������Ӧ��ȡ����Ӧ��
CH3COOCH2CH3��H2O����Ӧ����Ϊ������Ӧ��ȡ����Ӧ��
(4)�����Ȼ����ǻ����������Ʒ�Ӧ������������Ӧ�ķ���ʽΪ![]() ��2Na��H2����
��2Na��H2����![]() ��
��
(5)E����OH��COOH���ɷ���������Ӧ��������E�ɷ�Ӧ������Ԫ������Ӧ�ķ���ʽΪ2![]()
![]() 2H2O��
2H2O�� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ҵ��������������г������л��
��1����ͼ��ʾΪ___�������Ҵ��������������������ģ�͡�
��2���Ҵ��������еĹ����ŵ�����Ϊ___�������������еĹ����ŵ�����Ϊ___��
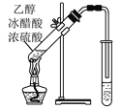
��3��д��ʵ�������Ҵ���ȡ��ȩ�Ļ�ѧ��Ӧ����ʽ___��
��4��д����ͼ��ʾװ�����Ҵ������ᷴӦ�Ļ�ѧ����ʽ��___���÷�Ӧ������Ϊ___��Ӧ���Ҳ�С�Թ���Ӧװ��___��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ȷ�������з�Ӧ�����ӷ���ʽΪ
A. �ô����ȥˮ����2H++CaCO3=Ca2++CO2��+H2O
B. ��������Ũ�����ϼ��ȣ�2H++FeS=H2S��+ Fe2+
C. ����������Һ�еμ�̼������Һ��2Al3++3![]() =Al2(CO3)3��
=Al2(CO3)3��
D. ������������Һ���չ�ҵ�����е�NO2��2NO2+2OH��=![]() +
+![]() + H2O
+ H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���谢���ӵ�������ֵΪNA������˵����ȷ���ǣ�������
A. ��״���£�2.24 Lˮ��������������ΪNA
B. ��27 g Al���뵽1 mol/L��NaOH��Һ�з�Ӧ��ת�Ƶĵ�����Ϊ3NA
C. 100 mL 2 mol/L��Na2CO3��Һ�к��е���������Ϊ0.6NA
D. 7.8 g Na2O2�������0.3NA������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ�Ǿ����Դ���⣺�Ӻ�ˮ����ȡʳ�κ���Ĺ������£�
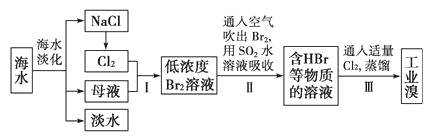
����˵���������
A.��ˮ�����ķ�����Ҫ�������������������ӽ�������
B.������ڵ��Ȼ�����һ��������ת��Ϊ��ѧ�ܵĹ���
C.������н�Br2��ԭΪBr����Ŀ���Ǹ�����Ԫ��
D.��ĸҺ�м���ʯ����ɵõ�Mg(OH)2����ҵ�ϳ��õ�����ڵ�Mg(OH)2����ȡþ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����100 mL NaOH��Һ��ͨ��һ������CO2���壬��ַ�Ӧ������������Һ����μ���0.2 mol/L�����ᣬ����CO2������������������֮���ϵ����ͼ��ʾ�������ж���ȷ���ǣ� ��

A. ԭNaOH��Һ��Ũ��Ϊ0.1 mol��L��1
B. ͨ��CO2�����Ϊ448 mL
C. ������Һ�����ʳɷֵ����ʵ���֮��Ϊn(NaOH)��n(Na2CO3)��1��3
D. ������Һ�����ʳɷֵ����ʵ���֮��Ϊn(NaHCO3)��n(Na2CO3) �� 2��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼװ�ÿ��Խ���ʵ�鲢�ܴﵽʵ��Ŀ�ĵ���
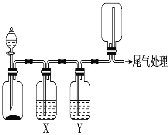
ѡ�� | ʵ��Ŀ�� | X���Լ� | Y���Լ� |
A | ��MnO2��Ũ������ȡ���ռ����������Cl2 | ����ʳ��ˮ | Ũ���� |
B | ��Cu��ϡ������ȡ���ռ����������NO | ˮ | Ũ���� |
C | CaCO3��ϡ������ȡ���ռ����������CO2 | ����NaHCO3��Һ | Ũ���� |
D | ��CaO��Ũ��ˮ��ȡ���ռ����������NH3 | NaOH��Һ | ��ʯ�� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���װ�Ǧ��(CH3NH3PbI3)������ȫ��̬���ѿ�����̫���ܵ�ص�����������CH3NH2��PbI2��HIΪԭ�Ϻϳɣ��ش��������⣺
(1)��ȡ�װ��ķ�ӦΪCH3OH(g)��NH3(g)![]() CH3NH2(g)��H2O(g)����H����֪�÷�Ӧ����ػ�ѧ���ļ����������£�
CH3NH2(g)��H2O(g)����H����֪�÷�Ӧ����ػ�ѧ���ļ����������£�
���ۼ� | C��O | H��O | N��H | C��N |
����/(kJ/mol) | 351.5 | 463 | 393 | 293 |
��÷�Ӧ����H��________kJ/mol��
(2)������Ӧ������ļ״���ҵ������ˮú���ϳɣ���ӦΪCO(g)��2H2(g) ![]() CH3OH(g) ��H<0����һ�������£���1 mol CO��2 mol H2ͨ���ܱ������н��з�Ӧ�����ı�ijһ�������(�¶Ȼ�ѹǿ)ʱ��CH3OH�����������(CH3OH)�仯������ͼ��ʾ��
CH3OH(g) ��H<0����һ�������£���1 mol CO��2 mol H2ͨ���ܱ������н��з�Ӧ�����ı�ijһ�������(�¶Ȼ�ѹǿ)ʱ��CH3OH�����������(CH3OH)�仯������ͼ��ʾ��

������˵�������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����________��
A.��ϵ��������ܶȱ��ֲ���
B.CO������������CH3OH�������������
C.��ϵ��CO��ת���ʺ�H2��ת�������
D.��ϵ��CH3OH������������ֲ���
��ƽ��ʱ��M��CH3OH���������Ϊ10%����CO��ת����Ϊ________��
��ijͬѧ��Ϊ��ͼ��Y���ʾ�¶ȣ�����Ϊ���жϵ�������______________________��
(3)ʵ���ҿ�����������Ǧ������ᷴӦ�Ʊ����ܵ�PbI2��ͬʱ����I2��д�������Ļ�ѧ��Ӧ����ʽ__________________��
(4)HI���Ʊ�����0.8molI2(g)��1.2molH2(g)����ij1L�ܱ������У���һ���¶��·�����Ӧ��I2(g)+H2(g)![]() 2HI(g)���ﵽƽ�⡣HI�����������ʱ��ı仯�������ʾ��
2HI(g)���ﵽƽ�⡣HI�����������ʱ��ı仯�������ʾ��
ʱ��(min) | 1 | 2 | 3 | 4 | 5 | 6 | 7/span> |
HI������� | 26% | 42% | 52% | 57% | 60% | 60% | 60% |
�ٸ÷�Ӧ��ƽ�ⳣ��K=_____________��
�ڷ�Ӧ�ﵽƽ�����7minʱ���������ѹ��Ϊԭ����һ�룬����ͼ�л���c(HI)��ʱ��仯������_______________��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com