
��1���й��Ŵ��Ĵ���֮һ�����ڻ�ҩ�����ı�ը��ӦΪ��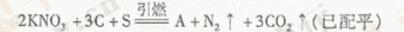

�ٳ�S�⣬����Ԫ�صĵ縺�ԴӴ�С����Ϊ ��
�����������У�A�ľ�������Ϊ �������Թ��ۼ��ķ��ӵ�����ԭ�ӹ���ӻ�����Ϊ ��
����֪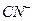 ��
��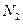 �ṹ���ƣ�����HCN������
�ṹ���ƣ�����HCN������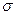 ����
����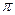 ����Ŀ֮��Ϊ ��
����Ŀ֮��Ϊ ��
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��ԭ������T��Q��2��T�Ļ�̬ԭ����Χ���ӣ��۵��ӣ��Ų�Ϊ ��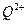 ��δ�ɶԵ������� ��
��δ�ɶԵ������� ��
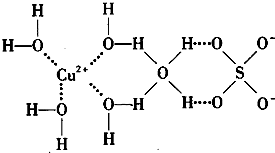
��3����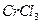 ��ˮ��Һ�У�һ�������´������Ϊ
��ˮ��Һ�У�һ�������´������Ϊ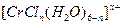 ��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��
��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��
����������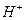 ���к͵ζ����������x��n,ȷ�������ӵ���ɡ�
���к͵ζ����������x��n,ȷ�������ӵ���ɡ�
����0��0015 mol 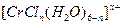 ����Һ����R-H��ȫ�������к����ɵ�
����Һ����R-H��ȫ�������к����ɵ�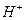 ��Ũ��Ϊ0��1200 mol
��Ũ��Ϊ0��1200 mol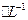 NaOH��Һ25��00 ml,��֪�������ӵĻ�ѧʽΪ ��
NaOH��Һ25��00 ml,��֪�������ӵĻ�ѧʽΪ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
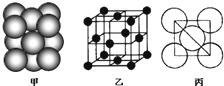
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1���й��Ŵ��Ĵ���֮һ--�ڻ�ҩ�����ı�ը��ӦΪ��2KNO3+3C+S
��1���й��Ŵ��Ĵ���֮һ--�ڻ�ҩ�����ı�ը��ӦΪ��2KNO3+3C+S
| ||
. |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ������
��2��ԭ������С��36��Ԫ��Q��T�������ڱ��мȴ���ͬһ������λ��ͬһ�壬��ԭ������T��Q��2��T�Ļ�̬ԭ����Χ���ӣ��۵��ӣ��Ų�Ϊ___________,Q2+��δ�ɶԵ�������_________________ ��
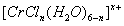 ��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��
��n��x��Ϊ���������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��  Rx[CrCl3(H2O)6-n]x+ +xH+
Rx[CrCl3(H2O)6-n]x+ +xH+�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com