
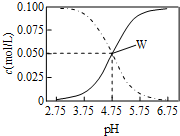
 25��ʱ���ڴ���ʹ����ƻ����Һ����c��CH3COOH��+c��CH3COO-��=0.1mol/L��c��CH3COOH����c��CH3COO-����pH�Ĺ�ϵ��ͼ���й�����Ũ��������ȷ���ǣ�������
25��ʱ���ڴ���ʹ����ƻ����Һ����c��CH3COOH��+c��CH3COO-��=0.1mol/L��c��CH3COOH����c��CH3COO-����pH�Ĺ�ϵ��ͼ���й�����Ũ��������ȷ���ǣ�������| A�� | pH=3.5��Һ�У�c��Na+��+c��H+��-c��OH-��+c��CH3COOH��=0.1mol/L | |
| B�� | pH=5.5��Һ�У�c��CH3COOH����c��CH3COO-����c��H+����c��OH-�� | |
| C�� | W���ʾ��Һ�У�c��Na+��+c��H+��=c��CH3COO-��+c��OH-�� | |
| D�� | ��W������ʾ��Һ��ͨ��0.05mol HCl���壨��Һ����仯�ɺ��ԣ���c��H+��=c��CH3COOH��+c��OH-�� |
���� ����ͼ��֪��������ҺpH��������Һ�д���Ũ�Ƚ��͡����������Ũ��������ʵ���Ǵ��������Ũ�ȡ������Ǵ���Ũ�ȱ仯��
A��pH=3.5����Һ�д��������غ㣬���������غ��жϣ�
B����pH=5.5ʱ��c��CH3COOH����c��CH3COO-����
C��W������ʾ����Һ��c��CH3COOH��=c��CH3COO-������Һ�д��ڵ���غ㣻
D�����������غ��� c��CH3COOH��+c��CH3COO-��=0.1 mol•L-1���ɵ���غ���c��Na+��+c��H+��=c��CH3COO-��+c��OH-��+c��Cl-����
��� �⣺����ͼ��֪��������ҺpH��������Һ�д���Ũ�Ƚ��͡����������Ũ��������ʵ���Ǵ��������Ũ�ȡ������Ǵ���Ũ�ȱ仯��
A��B��pH=3.5����Һ�д��������غ㣬���������غ㡢����غ��c��CH3COOH��+c��CH3COO-��=c��Na+��+c��H+��-c��OH-��+c��CH3COOH��=0.1mol•L-1����A��ȷ��
B����pH=5.5ʱ������ͼ��֪c��CH3COOH����c��CH3COO-������B����
C��W������ʾ����Һ��c��CH3COOH��=c��CH3COO-������Һ�д��ڵ���غ㣺c��Na+��+c��H+��=c��CH3COO-��+c��OH-������C��ȷ��
D����W������ʾ��Һ��ͨ��0.05molHCl���壬ԭ��ƽ�ⱻ���ƣ����������µ�ƽ�⣬��Һ�е���غ��ϵΪ��c��Na+��+c��H+��=c��CH3COO-��+c��OH-��+c��Cl-���������غ��ϵΪ��2c��Cl-��=c��CH3COO-��+c��CH3COOH��=0.1mol•L-1����2c��Na+��+2c��H+��=3c��CH3COO-��+2c��OH-��+c��CH3COOH����c��Na+��=0.05mol/L��c��CH3COOH��+c��CH3COO-��=0.1mol/L����c��H+���Tc��CH3COO-��+c��OH-������D����
��ѡAC��
���� ���⿼��������ϵĶ����жϼ�����Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ��Һ�д��ڵ�����ˮ�⡢ԭ���غ�������غ��ǽⱾ��ؼ���ע��������Һ���������ҺpH�Ĺ�ϵ���ܹ����õ���غ㡢�����غ㼰�ε�ˮ��ԭ���жϸ�����Ũ�ȴ�С��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A | |
| B |
| A�� | ��������ˮ�������ҷ�Ӧ | |
| B�� | �������������ǿ����Һ��Ӧ��������ǿ����Һ��Ӧ | |
| C�� | ��������Ļ�ѧʽΪBe��OH��3 | |
| D�� | ������ֻ����ǿ����Һ��������ǿ����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����״�ĵ�������ϡ���ᷴӦ����Ӧ�������ƺ�ˮ��Ӧ���� | |
| B�� | ���ڴ��������п���ȼ�գ�ȼ�ղ���Ͷ��ˮ�п��Էų����� | |
| C�� | ���ڴ����е�ȼ��ȼ�գ�ȼ�������IJ���Ͷ��ˮ�п��Էų����� | |
| D�� | ����״�ĵ�����Ͷ��ˮ�У����ҷ�Ӧ�����ɴ��������岢��������ȼ�պ�����ը |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
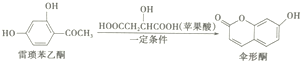
| A�� | ������ͪ�����ֺ��������� | |
| B�� | 1molɡ��ͪ������NaOH��Һ��Ӧ����������2mol NaOH | |
| C�� | ɡ��ͪ������ˮ | |
| D�� | ������ͪ��ɡ��ͪ���ܸ�FeCl3��Һ������ɫ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������� | B�� | ��ɫ���� | C�� | ��ͨ���� | D�� | �ӵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
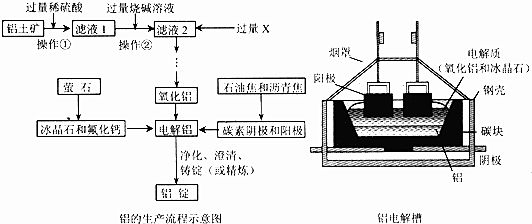
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
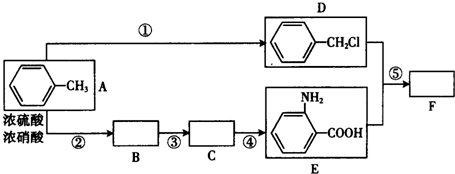
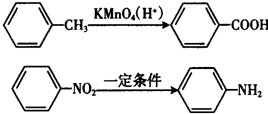
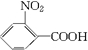 ��
�� ��
��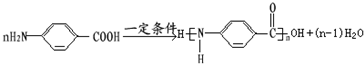 ��
��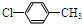 ��д�ṹ��ʽ����
��д�ṹ��ʽ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ױȺ��������� | |
| B�� | ���ױȺ����ȶ� | |
| C�� | �ñ仯Ϊ�����仯 | |
| D�� | �ñ仯�ı����Ǿɻ�ѧ�����ѣ��»�ѧ���γ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NH4+��NO3-��CO32-��Na+ | B�� | Na+��Ba2+��Mg2+��HCO3- | ||
| C�� | NO3-��K+��[Al��OH��4]-��OH- | D�� | NO3-��Mg2+��K+��Cl- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com