
����Ŀ����ͭ��������������к���Cu��Au(��)��PbSO4�����ʣ�ʪ����������������ۺ����õĹ���������ͼ��ʾ��
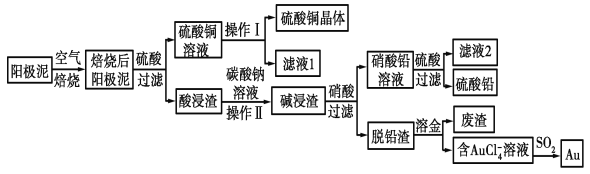
��1����⾫����ͭ����Ǧ�Ĵ�ͭʱ�����ҺӦ����________��Һ�����Һ�����ʱ�����ĵ缫��ӦʽΪ___________________________��Cu��2e��===Cu2����
��2����ɲ���������Ҫ�����У�__________________�����ˣ�ϴ�ӣ����
��3��д����SO2��ԭAuCl4-�����ӷ�Ӧ����ʽ____________________________��
��4��Ϊ�˼��ٷ�Һ�ŷš��������������Դ����ҵ�Ͻ���Һ1��������ͭ��Һ����ѭ����������ָ������ͼ����һ�����Ƶ�����________________________��
��5�������ӷ���ʽ��ʾ����̼������Һ�����ã�___________________________��[��֪298 Kʱ��Ksp(PbCO3)��1.46��10��13��Ksp(PbSO4)��1.82��10��8]������Һ��c(SO42-)=0.2mol/Lʱ��c(CO32-)=_______________mol/L�����������2λ��Ч���֣�
���𰸡�CuSO4 Pb-2e-+SO42-=PbSO4 ����Ũ������ȴ�ᾧ 3SO2+2AuCl4-+6H2O=2Au+3 SO42-+8Cl-+12H+ ����Һ2�ܽ������������������ܽ������� PbSO4��s��+CO32-(aq)![]() PbCO3��s��+SO42-(aq) 1.6��10-6
PbCO3��s��+SO42-(aq) 1.6��10-6
��������
��ͭ��������������к���Cu��Au(��)��PbSO4�����ʣ����պ����ͭ��Ϊ����ͭ��������Եõ�����ͭ��Һ������ͭ��Һ��������Ũ�������½ᾧ�����ˡ�ϴ��������������ͭ������Au(��)��PbSO4�����ʾ�̼���ƽ�ϴ��Ũ���������������˵õ�����Ǧ��Һ����Һ��������������Ǧ�������ٹ��˵õ�����Ǧ����Ǧ������Ҫ�ǽ�������ˮ�ܽ����õ�����AuCl4-����Һ��AuCl4-���Ա�SO2��ԭ�õ�Au���Դ˽��
��ͭ��������������к���Cu��Au(��)��PbSO4�����ʣ����պ����ͭ��Ϊ����ͭ��������Եõ�����ͭ��Һ������ͭ��Һ��������Ũ�������½ᾧ�����ˡ�ϴ��������������ͭ������Au(��)��PbSO4�����ʾ�̼���ƽ�ϴ��Ũ���������������˵õ�����Ǧ��Һ����Һ��������������Ǧ�������ٹ��˵õ�����Ǧ����Ǧ������Ҫ�ǽ�������ˮ�ܽ����õ�����AuCl4-����Һ��AuCl4-���Ա�SO2��ԭ�õ�Au��
(1)��⾫����ͭʱһ��������ͭ��Һ���������Һ����⾫���Ĵ�ͭ�������ᷢ��������Ӧ�����е���ͭ�ͻ����Ա�Cuǿ�Ľ������ᷢ���ܽ�����˴�ͭ�е�ͭ��Ǧ�ᷢ��ʧ���ӵ�������Ӧ���缫��ӦʽΪ��Pb-2e-+SO42-=PbSO4��
��ˣ�������ȷ������CuSO4��Pb-2e-+SO42-=PbSO4��
(2) ����I�IJ����Ǵ�����ͭ��Һ�л������ͭ��������˸ò���������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ�����
��ˣ�������ȷ����������Ũ�������½ᾧ��
(3) SO2��ԭAuCl4-�л�ԭ�������������Ƚ���ȷ����˺������Ʋ������������SO42-����ԭ������Au������������ԭ��Ӧ��ʧ�����غ���ȱ����ƽ��Ȼ����ݵ���غ�����ƽ����˵õ��ķ�Ӧ����ʽΪ��3SO2+2AuCl4-+6H2O=2Au+3 SO42-+8Cl-+12H+��
��ˣ�������ȷ������3SO2+2AuCl4-+6H2O=2Au+3 SO42-+8Cl-+12H+��
(4) ��Һ1���ڽᾧ����ͭʱʣ�µ���Һ��������������δ����������ͭ����˲���ǰ�������ͭ��Һ����ѭ���������ڳ��������������ƴ˴���������Ӧ���������еõ�����һ��Һ2������Һ2��������Ǧ��Һ�м���������������Ǧ����������������Ǧ��ʣ�µ���Һ������Һ��H+û�з�����Ӧ����˻��д�����������Һ�����Կ��Ѵ���Һ�����������ǰ��ļ�������ܽ�������
��ˣ�������ȷ���ǣ�����Һ2�ܽ������������������ܽ���������
(5) ͨ���Ƚ����ֳ������ܶȻ������Կ���̼��Ǧ������Ǧ�������������������Ǧ�м���̼�������������dz����ܽ�ת���ķ�Ӧ�����ӷ���ʽҪע�����״̬������ʽΪ��PbSO4��s��+CO32-(aq)![]() PbCO3��s��+SO42-(aq)��
PbCO3��s��+SO42-(aq)��
����Һ��c(SO42-)=0.2mol/Lʱ��c(CO32-)=![]() ��c(SO42-)=
��c(SO42-)=![]() ��0.2mol/L=1.6��10-6mol/L��
��0.2mol/L=1.6��10-6mol/L��
��ˣ�������ȷ������PbSO4��s��+CO32-(aq)![]() PbCO3��s��+SO42-(aq)��1.6��10-6��
PbCO3��s��+SO42-(aq)��1.6��10-6��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����Ԫ��R����������ϼ��븺���ϼ۴�����Ϊ6������������ȷ���ǣ� ��
A. R�ǵڢ�A��Ԫ��
B. R�����������ΪRO3
C. R����̬�⻯���ǿ�ȼ������
D. R����̬�⻯��������ˮ�Լ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ǰ��ԴΣ����һ��ȫ�������⣬��Դ������Ӧ����ԴΣ������Ҫ�ٴ롣
��1������������������Դ����Դ����������________(����ĸ)��
A��������չũ���������������Ľո�ת��Ϊ����Ч����Դ
B����������ú��ʯ�ͺ���Ȼ������������������������Դ����
C������̫���ܡ�ˮ�ܡ����ܡ������ܵ�����Դ������ʹ��ú��ʯ�͵Ȼ�ʯȼ��
D��������Դ���ģ��Ӵ���Դ���ظ�ʹ�á���Դ��ѭ������
��2�����ʯ��ʯī��Ϊ̼��ͬ�������壬��������������ʱȼ������һ����̼������������ʱ���ȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ��

����ͨ��״���£����ʯ��ʯī��Ƚϣ�________(�������ʯ������ʯī��)���ȶ���ʯī��ȼ������H��________��
��3����֪��N2��O2�����л�ѧ���ļ��ֱܷ���946kJ/mol��497kJ/mol��N2(g)��O2(g)=2NO(g)����H= +180.0kJ/mol��NO�����л�ѧ���ļ���Ϊ__________________kJ/mol��
��4���ۺ������й���Ϣ����д����CO��ȥNO��������Ⱦ������Ȼ�ѧ����ʽ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ũ�Ⱦ�Ϊ0.1 mol/L�������ΪV0��HX��HY��Һ���ֱ��ˮϡ�������V��pH��![]() ���仯��ϵ��ͼ��ʾ������������ȷ����
���仯��ϵ��ͼ��ʾ������������ȷ����

A��HX��HY�������ᣬ��HX�����Ա�HY����
B�������£���ˮ�������c(H+)��c��OH -����a<b
C����ͬ�¶��£����볣��K( HX)��a>b
D��![]() =3����ͬʱ��������Һ��������HX ��HY��H2O�Ļӷ�������
=3����ͬʱ��������Һ��������HX ��HY��H2O�Ļӷ�������![]() ��С
��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڵ�n���Ӳ��У�������Ϊԭ�ӵ�������Ӳ�ʱ��������ɵĵ�������n-1����ͬ��������Ϊԭ�ӵĴ����ʱ�����������n+1������ܶ�10������˵��Ӳ��ǣ� ��
A.K��B.M��
C.L��D.N��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��O3Ҳ��һ�ֺܺõ������������и�Ч���ྻ�����㡢���õ��ŵ㡣O3������ˮ����ˮ���ֽ⣬������[O]Ϊ������ԭ�ӣ��к�ǿ��ɱ���������������³�ѹ�·�����Ӧ���£�
��Ӧ����O3![]() O2��[O] ��H��0 ƽ�ⳣ��ΪK1��
O2��[O] ��H��0 ƽ�ⳣ��ΪK1��
��Ӧ���� [O]��O3![]() 2O2 ��H��0 ƽ�ⳣ��ΪK2��
2O2 ��H��0 ƽ�ⳣ��ΪK2��
�ܷ�Ӧ�� 2O3![]() 3O2 ��H��0 ƽ�ⳣ��ΪK��
3O2 ��H��0 ƽ�ⳣ��ΪK��
����������ȷ����( )
A. �����¶ȣ�K��С B. K��K1��K2
C. �ʵ����£����������Ч�� D. ѹǿ����K2��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������£����淴ӦN2(g)��3H2(g) ![]() 2NH3(g)����H��0���ﵽƽ�⣬�������ı������������й������������
2NH3(g)����H��0���ﵽƽ�⣬�������ı������������й������������
A. �Ӵ���v����v���������仯�����ұ仯�ı������
B. ��ѹv����v����������v������ı�������v������ı���
C. ���£�v����v������С����v����С�ı���С��v����С�ı���
D. ���������v����v����������v������ı�������v������ı���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij��Һ�к���![]() ��
��![]() ��Cl���������ӣ����ֻȡ��һ�θ���Һ(����ʹ�ù��˲���)�����ܰ������������μ��������
��Cl���������ӣ����ֻȡ��һ�θ���Һ(����ʹ�ù��˲���)�����ܰ������������μ��������
(1)Ӧ���ȼ���_____���ӣ�������Լ���ϡ���ᣬ��Ӧ�����ӷ���ʽΪ��_____________��
(2)�ټ���_____���ӣ�������Լ���_________����Ӧ�����ӷ���ʽΪ��__________________��
(3)������_____���ӣ�������Լ���_________����Ӧ�����ӷ���ʽΪ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧҪ̽��ȼ�յ������Ϳ����������ĺ���������������ʵ�顣
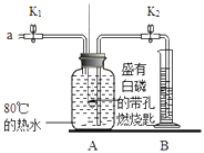
��ʵ�飩
�ټ��װ�õ������ԣ����������á�
����Aƿ�м���80����ˮ����ʢ�а��Ĵ���ȼ�ճ��ٽ�û��ˮ�У�����ƿ�������ײ�ȼ�ա�
�۴�K1��K2����a����Aƿ�й����������ƿ�е�Һ�����ȼ�ճײ�ʱ���ر�K1��K2����ʱ������ͲB��ˮ�����Ϊ200mL���۲쵽Aƿ�еİ���ȼ�ա�
��1���ԱȲ���ں͢۵������֪����ȼ��ȼ�յ�����֮һ��___������ȼ�յķ�Ӧ����ʽΪ_____��
��2����װ����ȴһ��ʱ���K2������۲쵽____��˵�����������������Լռ1/5�����ȷ����װ�����������ã�������ȼ�ճ�λ��©�������______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com