
����Ŀ����ǰ��ԴΣ����һ��ȫ�������⣬��Դ������Ӧ����ԴΣ������Ҫ�ٴ롣
��1������������������Դ����Դ����������________(����ĸ)��
A��������չũ���������������Ľո�ת��Ϊ����Ч����Դ
B����������ú��ʯ�ͺ���Ȼ������������������������Դ����
C������̫���ܡ�ˮ�ܡ����ܡ������ܵ�����Դ������ʹ��ú��ʯ�͵Ȼ�ʯȼ��
D��������Դ���ģ��Ӵ���Դ���ظ�ʹ�á���Դ��ѭ������
��2�����ʯ��ʯī��Ϊ̼��ͬ�������壬��������������ʱȼ������һ����̼������������ʱ���ȼ�����ɶ�����̼����Ӧ�зų���������ͼ��ʾ��

����ͨ��״���£����ʯ��ʯī��Ƚϣ�________(�������ʯ������ʯī��)���ȶ���ʯī��ȼ������H��________��
��3����֪��N2��O2�����л�ѧ���ļ��ֱܷ���946kJ/mol��497kJ/mol��N2(g)��O2(g)=2NO(g)����H= +180.0kJ/mol��NO�����л�ѧ���ļ���Ϊ__________________kJ/mol��
��4���ۺ������й���Ϣ����д����CO��ȥNO��������Ⱦ������Ȼ�ѧ����ʽ��_______________________________��
���𰸡�ACD ʯī -393.5kJ��mol-1 631.5 2NO(g)+2CO(g)=N2(g)+2CO2(g)����H= -746.0kJ��mol-1
��������
��1��ֻҪ�ܼ��ٻ�ʯȼ�ϵ���Դ�����ö���������Դ����������2��������������Խ�ߣ�����Խ���ȶ�������ͼ���ж�ʯī��ȼ���ȣ����ݼ�ֵ�����ж����ɵ�������ɣ�����Ȼ�ѧ����ʽ����õ�����3���ɼ��������յ�������ȥ�¼������ͷŵ�����ֵ��Ϊ��ѧ��Ӧ�����յ����������N2��O2�����л�ѧ���ļ��ֱܷ���946kJ/mol��497kJ/mol�������Ȼ�ѧ����ʽN2(g)+O2(g)=2NO(g)����H= +180kJ/mol���Լ���NO�����л�ѧ���ļ��ܣ���4�����ø�˹���ɽ����֪�Ȼ�ѧ����ʽ���㷴Ӧ�ȣ���д���Ȼ�ѧ����ʽ��
��1��ֻҪ�ܼ��ٻ�ʯȼ�ϵ���Դ�����ö���������Դ��������B����������ú��ʯ�ͺ���Ȼ�������ܼ��ٻ�ʯȼ�ϵ����ã��ʴ���A��C��D�ܼ��ٻ�ʯȼ�ϵ����ã���ȷ����ѡACD��
��2��ͼ��������ʯ��������ʯī������Խ��Խ�ȶ�������˵��ʯī�ȶ���ͼ�����1molʯī��ȫȼ������1mol������̼�ų�������Ϊ393.5kJ����ʯī��ȼ����Ϊ��H= -393.5 kJ/mol��
��3����H=��Ӧ����ܺ�-��������ܺͣ�����N2(g)��O2(g)= 2NO(g)����H= +180.0 kJ/mol����946kJ/mol+ 497kJ/mol-2Q(N-O)=180.0kJ/mol���Q(N-O)=631.5kJ/mol��
��4����֪��C(ʯī,s)+O2(g)=CO2(g) ����H= -393.5kJ/mol��C(ʯī,s)+1/2O2(g)=CO(g)����H= -110.5kJ/mol��N2(g)+O2(g)=2NO(g)����H= +180kJ/mol���ɸ�˹����������ʽ����2-����2-�۵�2NO(g)+2CO(g)=N2(g)+2CO2(g)����H= -746.0kJ/mol��


 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������(K2FeO4)��һ���͡���Ч�����Ķ��ˮ��������
�����ϣ�K2FeO4Ϊ��ɫ���壬����KOH��Һ������ǿ�����ԣ������Ի�������Һ�п��ٲ���O2���ڼ�����Һ�н��ȶ���
��1���Ʊ�K2FeO4��
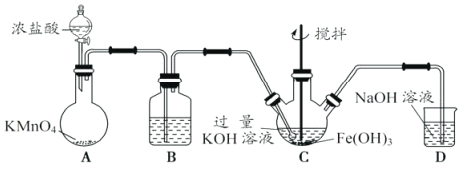
��AΪ��ȡ��������װ�ã�����ʢ��Ũ�������������Ϊ___________��
�ڳ���װ��B�е��Լ�Ϊ___________��
��CΪ�Ʊ�K2FeO4װ�ã�KOH��Һ������ԭ����___________��
��DΪβ������װ�ã�������Ӧ�����ӷ���ʽΪ___________��
��2��̽�� K2FeO4�����ʡ�ȡC����ɫ��Һ������ϡ���ᣬ�����S��ɫ���壬����Һa�������������к���Cl2��Ϊ֤��K2FeO4�ܷ�����Cl��������Cl2��������·�����
����I | ȡ������Һa���μ�KSCN��Һ����������Һ�ʺ�ɫ�� |
������ | ��KOH��Һ���ϴ��C�����ù��壬����KOH��Һ��K2FeO4�ܳ����õ���ɫ��Һb��ȡ����b���μ����ᣬ��Cl2������ |
���ɷ���I����Һ����֪a�к���___________�������ӵIJ���___________(������������������)�ж�һ������K2FeO4��Cl����ԭ���γɵġ�
�ڷ�������KOH��Һϴ�ӵ�Ŀ����___________���������ó������ԣ�Cl2___________FeO42��(����>������<��)
��3��ʹ��ʱ����ͨ���ⶨ������صĴ������ж����Ƿ���ʡ�K2FeO4��������Һ�з�Ӧ������_______ FeO42��+______H+===_______O2��+________Fe3++________(��ƽ������������ӷ���ʽ)________����ȡC��ϴ�Ӳ��������Ʒ������10g������ϡ���ᣬ�ռ���0.672L����(��״����)������Ʒ�и�����ص���������ԼΪ___________��(������������0.1%)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��ϡ��Һ�ǿ���ǿ����кͷ�Ӧ����1 molҺ̬ˮʱ��Ӧ�Ƚ����к��ȡ���������ͼװ�ý����к��ȵIJⶨ����ش��������⣺

(1)ͼ��δ������ʵ��������________________��________________��
(2)��һ���������к��Ȳⶨʵ�飬�¶ȼ���ʹ��________�Ρ�
(3)ʵ��ʱ����0.50 mol��L��1��������뵽0.55mol��L��1��NaOH��Һ�У�������Һ�������Ϊ50 mL������Һ���ܶȾ�Ϊ1 g /cm3��������Һ�ı�����c=4.18 J /(g�� oC)��ʵ�����ʼ�¶�Ϊt1 oC����ֹ�¶�Ϊt2 oC������¶ȱ仯�������£�
��� | ��Ӧ�� | ��ʼ�¶�t1/ oC | ��ֹ�¶�t2/ oC | �к��� |
�� | HCl��NaOH | 14.8 | 18.3 | ��H1 |
�� | HCl��NaOH | 15.1 | 19.7 | ��H1 |
�� | HCl��NaOH | 15.2 | 18.5 | ��H1 |
�� | HCl��NH3��H2O | 15.0 | 18.1 | ��H2 |
���Լ�����������ʵ�������к�����H1��______________��
��ijС��ͬѧΪ̽��ǿ���������ϡ��Һ��Ӧʱ�������仯���ֶ�����һ��ʵ��ܣ���0.55mol��L��1��ϡ��ˮ����NaOH��Һ������¶ȵı仯��������У��Լ��㷴Ӧ����H2��____________��
������ʵ���������ԭ����___________________________________________��
��д��HCl��NH3�� H2O��Ӧ���Ȼ�ѧ����ʽ��_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ��ˮ�ĵ���ɴﵽƽ�⣺H2O![]() H++OH- ��H>0������������ȷ����
H++OH- ��H>0������������ȷ����
A. ��ˮ�м���ϡ��ˮ��ƽ�������ƶ���c(OH-)����
B. ��ˮ���ȣ�Kw����pH����
C. ��ˮ�м�������CH3COONa���壬ƽ�������ƶ���c (H+)����
D. ��ˮ�м������������������ƣ�c(H+)����Kw����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����ˮϵ�����ӵ�ع���ԭ������ͼ��ʾ��TiO2��缫��ʹ�����̫�������³�磬���ʱ Na2S4ת��Ϊ Na2S������˵����ȷ����
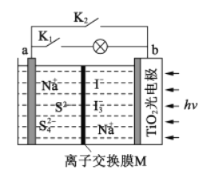
A. ���ʱ��̫����ת��Ϊ��ѧ�ܣ���ѧ����ת��Ϊ����
B. �ŵ�ʱ��a��Ϊ����
C. ���ʱ�������ĵ缫��ӦʽΪ3I--2e-=I3-
D. M����ʹ�������ӽ���Ĥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ⶨij����ϡ����Ʒ�з�Ԫ�ص�����������ij��ѧ��ȤС�����������ʵ�飬���ø�����(�߷е���)����Ʒ�еķ�Ԫ��ת��Ϊ������(�ͷе���)����������ͨ���ζ�������ʵ��װ����ͼ��ʾ��
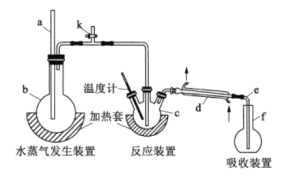
(1)a��������__________������d��������__________��
(2)���װ�������ԣ���b��f�м�ˮ��ʹˮ��û����a��eĩ�ˣ�__________��ֹͣ���ȣ�����e����һ���ȶ���ˮ����˵��װ�����������á�(��ȫ���ϲ��������еĿ�ȱ����)
(3)c�м���һ�������������Һ��0.100g����ϡ����Ʒ��f��ʢ�еμӷ�̪�� NaOH��Һ������b��c��ʹb�в�����ˮ��������c��
�����������пɴ�����������________(�����)��
a.���� b.���� c.���� d.����
�����۲쵽f����Һ��ɫ��ȥ����Ҫ��f�м�ʱ����NaOH��Һ�������ʹʵ����ƫ�ͣ�ԭ����___________________��ʵ���г���HF�����⣬���ܻ�������SiF4(��ˮ��)�������ɣ���ʵ��������Ӱ�죬ԭ����__________(�û�ѧ����ʽ��ʾ)��
(4)�����Һ�м���25.00mL0.100mol/LLa(NO3)3��Һ���õ�LaF3����������0.100mol/LEDTA����Һ�ζ�ʣ��La3+( La3+��EDTA��1��1���)������EDTA����Һ22.00mL�������ϡ����Ʒ�з�Ԫ�ص���������Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ӻ�ˮ����Ҫ��ˮ���Ȼ��ƣ�����������þ���ӵȣ�����ȡþ���ɰ����²�����У�
�ٰѱ��ǣ���Ҫ�ɷ�̼��ƣ��Ƴ�ʯ���飨�������Ƶ�����Һ����
��������ĺ�ˮ�м���ʯ���飬���������ˡ�ϴ�ӳ������Ҫ��������þ����
�۽������������ᷴӦ���ᾧ���ˡ���HCl�����Χ�и������õ��Ȼ�þ���
�ܽ��õ��IJ������ڵ��õ�þ��
����˵������ȷ����
A. ��ˮ�м����������������ɫ����
B. ��������漰�������ӷ�Ӧ����ʽΪ2OH��+Mg2��=Mg(OH)2��
C. �٢ڢ۲����漰���ϡ��ֽ���ֽⷴӦ
D. �ڢܲ��ķ�Ӧ���ڷֽⷴӦҲ��������ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ��������������к���Cu��Au(��)��PbSO4�����ʣ�ʪ����������������ۺ����õĹ���������ͼ��ʾ��
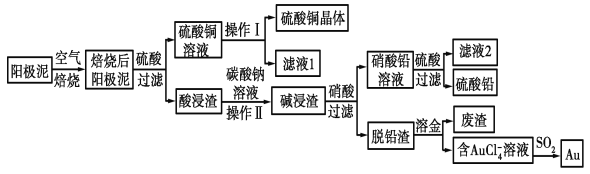
��1����⾫����ͭ����Ǧ�Ĵ�ͭʱ�����ҺӦ����________��Һ�����Һ�����ʱ�����ĵ缫��ӦʽΪ___________________________��Cu��2e��===Cu2����
��2����ɲ���������Ҫ�����У�__________________�����ˣ�ϴ�ӣ����
��3��д����SO2��ԭAuCl4-�����ӷ�Ӧ����ʽ____________________________��
��4��Ϊ�˼��ٷ�Һ�ŷš��������������Դ����ҵ�Ͻ���Һ1��������ͭ��Һ����ѭ����������ָ������ͼ����һ�����Ƶ�����________________________��
��5�������ӷ���ʽ��ʾ����̼������Һ�����ã�___________________________��[��֪298 Kʱ��Ksp(PbCO3)��1.46��10��13��Ksp(PbSO4)��1.82��10��8]������Һ��c(SO42-)=0.2mol/Lʱ��c(CO32-)=_______________mol/L�����������2λ��Ч���֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪:��2H2(g)+O2(g)![]() 2H2O(l)����H=-571.6 kJ��mol-1
2H2O(l)����H=-571.6 kJ��mol-1
��2CH3OH(l)+3O2(g)![]() 2CO2(g)+4H2O(l)����H=-1 452 kJ��mol-1
2CO2(g)+4H2O(l)����H=-1 452 kJ��mol-1
��H+(aq)+OH-(aq)![]() H2O(l)����H=-57.3 kJ��mol-1
H2O(l)����H=-57.3 kJ��mol-1
����˵����ȷ����
A. H2(g)��ȼ����Ϊ142.9 kJ��mol-1
B. ͬ������H2(g)��CH3OH(l)��ȫȼ��,H2(g)�ų���������
C. 1/2H2SO4(aq)+1/2Ba(OH)2(aq)![]() 1/2BaSO4(s)+H2O(l)����H=-57.3 kJ��mol-1
1/2BaSO4(s)+H2O(l)����H=-57.3 kJ��mol-1
D. 3H2(g)+CO2(g)![]() CH3OH(l)+H2O(l)����H=+131.4 kJ��mol-1
CH3OH(l)+H2O(l)����H=+131.4 kJ��mol-1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com