
��ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪��
�ٷ�ӦC+G B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
��I��һ�ֳ������������壬����E���Է�����Ӧ��2E+I 2F+D��F��EԪ�ص���������Ϊ60%��
2F+D��F��EԪ�ص���������Ϊ60%��

�ش����⣺
��1�����з�Ӧ�Ļ�ѧ����ʽΪ��________________________________________��
��2��1.6 g G�������ᣬ�õ�����Һ��ͭ����ȫ��Ӧ������������ͭ�۵�������д�����ӷ���ʽ�ͼ�����̣���__________________________________________��
��3��C�����NaOH��Һ��Ӧ�����ӷ���ʽΪ��___________________________����Ӧ����Һ�����������I��Ӧ�����ӷ���ʽΪ��_________________________��
��4��E��I��ȼ�չ۲쵽�������ǣ�____________________________________��
��1��
��2��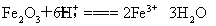

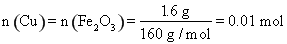 ��������ͭ�۵�����Ϊ0.01 mol��64 g/mol=0.64 g
��������ͭ�۵�����Ϊ0.01 mol��64 g/mol=0.64 g
��3��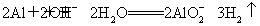

��4��þ������ȼ�գ����ɰ�ɫ��ĩ����Ӧ���ڱڸ��ź�ɫ����
��������������Ҫ����Mg��Al��Fe���仯��������ʼ������йصĻ�ѧ����ʽ�ļ��㡣����ʱע��Ѱ��ͻ�ƿڣ������ȷ�ӦӦ��������ĺ��ӡ�CO2���������������ȡ�
��1�����ȷ�ӦӦ��������ĺ��ӣ��ɢٿ�֪CΪAl��GΪFe2O3��BΪFe��HΪAl2O3����Ӧ�Ļ�ѧ����ʽΪ2Al��Fe2O3 2Fe��Al2O3��CO2��������������壬�ɢڿ�֪EΪMg��IΪCO2��FΪMgO��DΪC�����Ͽ�֪AΪO2��
2Fe��Al2O3��CO2��������������壬�ɢڿ�֪EΪMg��IΪCO2��FΪMgO��DΪC�����Ͽ�֪AΪO2��
��2��Fe2O3�����ᷴӦ�����ӷ���ʽΪFe2O3��6H+=2Fe3+��3H2O��FeCl3��ͭ����ȫ��Ӧ�����ӷ���ʽΪ2Fe3+��Cu=2Fe2+��Cu2+����֪n��Cu��=n��Fe2O3��=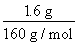 =0.01 mol,��ͭ�۵�����Ϊ0.01 mol��64 g/mol=0.64 g��
=0.01 mol,��ͭ�۵�����Ϊ0.01 mol��64 g/mol=0.64 g��
��3��Al�����NaOH��Һ��Ӧ�����ӷ���ʽΪ��2Al��2OH-��2H2O= ��
��
3H2������Ӧ����ҺΪNaAlO2�������CO2��Ӧ�����ӷ���ʽΪ�� ��
��
��4��Mg��CO2��ȼ�յĻ�ѧ����ʽΪ��2Mg+CO2 2MgO��C���۲쵽��������þ������ȼ�գ����ɰ�ɫ��ĩ����Ӧ���ڱڸ��ź�ɫ���塣
2MgO��C���۲쵽��������þ������ȼ�գ����ɰ�ɫ��ĩ����Ӧ���ڱڸ��ź�ɫ���塣


 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 5-2Ԫ�����ڱ� Ԫ����������ϰ���������棩 ���ͣ�ѡ����
����Ԫ�����ڱ���Ԫ�������ɣ������ƶ���ȷ���ǣ� ��
A��H3BO3�����Ա�H2CO3��ǿ
B��Mg��OH��2�ļ��Ա�Be��OH��2��ǿ
C��HCl��HBr��HI�����ȶ���������ǿ
D����M+��R2-�ĺ�����Ӳ�ṹ��ͬ����ԭ��������R��M
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 4-3������Ҫ��������ϰ���������棩 ���ͣ�ѡ����
SO2�dz����Ĵ�����Ⱦ��֮һ�������й�SO2��˵������ȷ���ǣ� ��
A.SO2�Ի�������ҪӰ�����γ�����
B.����Ȼ����ˮú�������ȼ�ϴ���ú̿������ȼ�ϻ���ú�м�����ʯ�Һ�ȼ�գ����Լ���SO2���ŷ���
C.���᳧ʹ��V2O5���������ӿ�SO2��ת�����ʣ����Լ���SO2���ŷ���
D.ֲ�����֣��̻����������ڼ��ٿ�����SO2�ĺ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 4-1���ǽ������ϵ�����-����ϰ���������棩 ���ͣ�ѡ����
������CO2����ͨ��ˮ������Na2SiO3��Һ���У�Ȼ��������ɣ����ڸ����³�����գ����õ��Ĺ��������ǣ� ��
A.Na2SiO3 B.Na2CO3��Na2SiO3 C.Na2CO3��SiO2 D.SiO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 4-1���ǽ������ϵ�����-����ϰ���������棩 ���ͣ�ѡ����
������������̼�����Ʒ�ĩ���Ƿ����̼���Ƶ�ʵ�鷽���ǣ� ��
A�����ȣ�����������ų�
B���μ����ᣬ����������ų�
C������ˮ�μ�ϡ���Ȼ�����Һ�����ް�ɫ��������
D������ˮ�μӳ���ʯ��ˮ�����ް�ɫ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 3-3��������Ҫ��������ϰ���������棩 ���ͣ�ѡ����
A��B��C����ѧ�������ֵ��ʣ�A��B��C����������֮��ķ�Ӧ��ϵ��ͼ��ʾ������B��D��E��F��ˮ��Һ�����ԡ���D+C E+F�ҳ�����ֻ��BΪ��̬����A��B��C�ֱ�Ϊ�� ��
E+F�ҳ�����ֻ��BΪ��̬����A��B��C�ֱ�Ϊ�� ��

A.Fe��Cl2��Cu B.Fe��Cl2��H2 C.Cu��Cl2��Fe D.Fe��Cl2��Mg
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 3-2��������Ҫ��������ϰ���������棩 ���ͣ������
���ڽ������ӵ�ij�ֺ�������ҩƤ�ɴ���ʯ��ˮ�ࡢ���������ƶ��ɡ�
��1��Al��ԭ�ӽṹʾ��ͼΪ____________��Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ____________��
��2��30Si��ԭ�ӵ�������Ϊ________________________��
��3��Al3+��Yn-�ĵ�������ͬ��Y�������Ԫ�ص��⻯���ˮ��Һ�������ԣ�������⻯���зе���͵���____________________________________��
��4�����ӹ����У�ҩƤ�ڸ����²�����������ʹ�����������������壬��������____________��
��5���������������36.0 g������Fe2O3��Al2O3��SiO2������������ϡ���ᣬ����õ�11.0 g���壻��Һ�м������NaOH��Һ������õ�21.4 g���壻���������Al2O3����������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 3-1�Ƽ�����Ҫ��������ϰ���������棩 ���ͣ�ѡ����
��8 g Na2O2��Na2O��Na2CO3��NaOH�Ļ������200 g��������Ϊ3��65%������ǡ�÷�Ӧ��������Һ�����յù�������Ϊ�� ��
A��8 g B��15��5 g C��11��7 g D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�껯ѧ�߿��ܸ�ϰ��ʱ���� 2-1���ʵķ�����ϰ���������棩 ���ͣ�ѡ����
���з���ͼ��ʾ�Ĺ�ϵ��ȫ��ȷ���ǣ� ��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com