
����Ŀ����ʽ�����[Ca10(PO4)6(OH)2],��ҵ�Ͻ��ǻ���ʯ������ʯ,���˵������бȽϼ�Ӳ�����ʣ���ˮ���������ܽ⣬������������Ҫԭ�������Ҳ�Ʒ���ʯ,���Ļ�ѧʽΪCa10(PO4)6F2,�����������Ե���Ҫ�ɷ֡�
�ش��������⣺
(1)��̬��ԭ�ӵļ۲�����Ų�ʽΪ____________����̬��ԭ�ӵ�δ�ɶԵ�����Ϊ______________��
(2)��ʽ������к���������ӵ�����ԭ���ӻ�������______________________,����������ӵĿռ乹��Ϊ______________��
(3)�������Һ�д��ڣ�2HF![]() H2F2,H2F2ΪһԪ�ᣬ���������Һ�к�����������______________(�����ӷ���)��������д���H2F2��ԭ����______________________________________________����H2F+��Ϊ�ȵ�����ķ�����________________(д��һ������)��
H2F2,H2F2ΪһԪ�ᣬ���������Һ�к�����������______________(�����ӷ���)��������д���H2F2��ԭ����______________________________________________����H2F+��Ϊ�ȵ�����ķ�����________________(д��һ������)��
(4)O��F��P�ĵ縺����С�����˳��Ϊ______________��
(5)NH3��PH3�Ĺ�������,NH3�ļ����Դ���PH3,��ԭ�ӽṹ�ǶȽ�����ԭ��________________��
(6)CaF2������ͼ��ʾ��λ�����ڵ����ӵ���λ��Ϊ______________��

��֪�������ƾ����ܶ�Ϊ��g��cm-3,NA���������ӵ�������ֵ��
�����ƾ�����Ca2+��F-֮������˼��(d)Ϊ______________pm(ֻҪ���г�����ʽ����)��
���𰸡�4s23sp3��������F-��HF2-HF����֮��������H2O��H2S��H2Se��P<O��FNԭ�Ӱ뾶С��P��H-N��֮���ų�������H-P��4![]()
��������
(1)��̬��ԭ�ӵĺ�������Ų�ʽΪ��1s22s22p63s23p64s2������۲������Ϊ��4s2����̬��ԭ�ӵĺ�������Ų�ʽΪ��1s22s22p63s23p3����3p�����������δ�ɶԵ��ӣ��ʴ�Ϊ��4s2��3��(2)��ʽ������к����������ΪPO43-��������ԭ�ӵļ۲���Ӷ���=![]() ��5+3+0��=4�������ӻ�����Ϊsp3 ���ռ乹��Ϊ�������壬�ʴ�Ϊ��sp3���������壻(3)���������Һ�д��ڣ�2HF
��5+3+0��=4�������ӻ�����Ϊsp3 ���ռ乹��Ϊ�������壬�ʴ�Ϊ��sp3���������壻(3)���������Һ�д��ڣ�2HF![]() H2F2 ������Һ�д���HF��H2F2����һԪ�ᣬ��������F��HF2-��֮���Ի����H2F2������ΪHF���Ӽ���������H2F+ �ļ۵�������Ϊ8����ԭ������Ϊ3������֮��Ϊ�ȵ�����ķ�����H2O��H2S��H2Se�ȣ��ʴ�Ϊ��F-��HF2-��HF����֮����������H2O��H2S��H2Se�ȣ�(4)����N��O��P����Ԫ�صķǽ����Ե�ǿ����֪��縺����С�����˳��ΪP<O��F ���ʴ�Ϊ��P<O��F ��(5)�� Nԭ�Ӱ뾶С��P��H-N��֮���ų�������H-P������NH3�ļ����Դ���PH3 ���ʴ�Ϊ��Nԭ�Ӱ뾶С��P��H-N��֮���ų�������H-P����(6)�ɾ����ṹ����λ�����ڵ�����ΪF-����֮�Ⱦ��������Ca2+Ϊ4����������λ��Ϊ4�������к�����������Ϊ8�������ӵ���ĿΪ8��1/8+6��1/2=4����������m=(19��8+40��4)/NA���������V=a3�������ܶ���=m/V=312/(NA��a3)����a3=312/��NAcm3��������Ʒ����Ca2+��F-֮������˼��(d)Ϊ
H2F2 ������Һ�д���HF��H2F2����һԪ�ᣬ��������F��HF2-��֮���Ի����H2F2������ΪHF���Ӽ���������H2F+ �ļ۵�������Ϊ8����ԭ������Ϊ3������֮��Ϊ�ȵ�����ķ�����H2O��H2S��H2Se�ȣ��ʴ�Ϊ��F-��HF2-��HF����֮����������H2O��H2S��H2Se�ȣ�(4)����N��O��P����Ԫ�صķǽ����Ե�ǿ����֪��縺����С�����˳��ΪP<O��F ���ʴ�Ϊ��P<O��F ��(5)�� Nԭ�Ӱ뾶С��P��H-N��֮���ų�������H-P������NH3�ļ����Դ���PH3 ���ʴ�Ϊ��Nԭ�Ӱ뾶С��P��H-N��֮���ų�������H-P����(6)�ɾ����ṹ����λ�����ڵ�����ΪF-����֮�Ⱦ��������Ca2+Ϊ4����������λ��Ϊ4�������к�����������Ϊ8�������ӵ���ĿΪ8��1/8+6��1/2=4����������m=(19��8+40��4)/NA���������V=a3�������ܶ���=m/V=312/(NA��a3)����a3=312/��NAcm3��������Ʒ����Ca2+��F-֮������˼��(d)Ϊ![]() cm ,��
cm ,��![]() pm���ʴ�Ϊ��4��
pm���ʴ�Ϊ��4��![]() ��
��


 ������ȫ��������ϵ�д�
������ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�������£�ͨ�����з�Ӧ��ʵ��ȼú��������Ļ��գ�
SO2(g)+2CO(g)![]() 2CO2(g)+S(l)��H��0 ����Ӧ�ں��ݵ��ܱ������н��У������й�˵����ȷ����
2CO2(g)+S(l)��H��0 ����Ӧ�ں��ݵ��ܱ������н��У������й�˵����ȷ����
A. ƽ��ǰ�����ŷ�Ӧ�Ľ��У�������ѹǿʼ�ղ���
B. ƽ��ʱ�������������䣬�����������Ӧ���ʼӿ�
C. ƽ��ʱ�������������䣬�����¶ȿ����SO2��ת����
D. �����������䣬ʹ�ò�ͬ�������÷�Ӧƽ�ⳣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(10��)������X��һ�����ϣ��ɲ�����ϩ��ױ�Ϊ��Ҫԭ�ϣ�������·�ߺϳɣ�

��֪��RX![]() ROH��RCHO��CH3COOR��
ROH��RCHO��CH3COOR��![]() RCH��CHCOOR��
RCH��CHCOOR��
��ش�
��1��E�й����ŵ������� ��
��2��B��D��F�Ļ�ѧ����ʽ ��
��3��X�Ľṹ��ʽ ��
��4�����ڻ�����X������˵����ȷ���� ��
A���ܷ���ˮ�ⷴӦ B������Ũ���ᷢ��ȡ����Ӧ
C����ʹBr2/CCl4��Һ��ɫ D���ܷ���������Ӧ
��5�����л�����������F��ͬ���칹����� ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�У�ˮ����ԭ������
A. Mg��2H2O![]() Mg(OH)2��H2��
Mg(OH)2��H2��
B. 2F2+2H2O�T4HF+O2
C. Cl2��H2O![]() HCl+HClO
HCl+HClO
D. 2Na2O2+2H2O�T4NaOH+O2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���Ľṹ���ü���ʽ��ʾ����CH3-CH=CH-CH3�ɱ�ʾΪ![]() ����һ���л���X�ļ���ʽ��ͼ��ʾ��
����һ���л���X�ļ���ʽ��ͼ��ʾ��

(1)X�ķ���ʽΪ_______________________��
(2)�л���Y��X��ͬ���칹�壬�����ڷ����廯�����Y�Ľṹ��ʽ��_____________��
(3)Y��һ�������¿ɷ�����Ӧ���ɸ߷��ӻ�����÷�Ӧ�Ļ�ѧ����ʽ��_________________��
(4)Y��������ˮ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽ��____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪���������ڿ������ܱ����������������ơ�ij��ȤС��Ϊ�����������ƹ����Ƿ���ʼ�̽��������������ʣ����������ʵ��:

I������Na2SO3�Ƿ����
��1����Բ����ƿA�м������Һ1.0 mol/LNa2SO3��Һ50mL��ҺaΪ_______����ҺbΪ_______��
��2����װ��A�еμ�������Һa��Na2SO3��Ӧ��ȫ����װ��AʹSO2��ȫ�ݳ���ʵ��ǰ����Cװ������2.4g����Na2SO3��Һ���ʵ���Ũ��Ϊ_______mol/L�����ж�Na2SO3�����Ƿ����______�����ǻ����

II����������װ���Ʊ�����SO2�����ʵ��Ƚ�H2SO3�� H2CO3��H2SiO3������ǿ������̽��SO2�Ļ�ѧ���ʡ�
��1���Լ�X��___________���Լ�Y��___________���Լ�Z��___________��
��2���ر�ֹˮ��b����ֹˮ��a����װ����ͨ��SO2�����۲쵽_________����֤��H2CO3���Ա�H2SiO3ǿ��
��3���ر�ֹˮ��a����ֹˮ��b������ͨ��SO2����ˮ��ɫ��˵��SO2����_______��д����Ӧ�����ӷ���ʽ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й����ʵ�ת����ϵ����ͼ��ʾ(����������ͷ�Ӧ��������ȥ)��AΪ������Ԫ����ɵĹ��壬���ǽ�����������Ϊ39.13%��D��E��L��Ϊ��ɫ���壬E��L������ˮ��FΪ����������G��I��J��ɫ��Ӧ�Ի�ɫ��MΪ����ɫ�Ĺ��塣
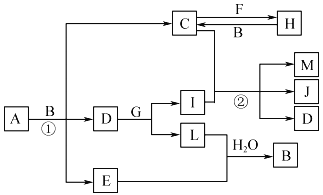
��ش��������⣺
��1��E�Ļ�ѧʽΪ___________________��
��2��G�ĵ���ʽΪ___________________��
��3��д����Ӧ�ٵĻ�ѧ����ʽ��_______________________________��
��4��д����Ӧ�ڵ����ӷ���ʽ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴Ӧ��aA��g��+bB��g��![]() cC��g��+dD��g��������ͼ�ش�
cC��g��+dD��g��������ͼ�ش�

��1��ѹǿp1��p2 �����С����
��2����a+b���ȣ�c+d�� �����С����
��3���¶�t1��t2�� ����ߡ��͡���
��4������ӦΪ �ȷ�Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ��CuFeS2������ͭ������Ҫ�����ұ����ͭ��Ĺ����У�����һ����Ӧ�ǣ�2Cu2O+ Cu2S![]() 6Cu+SO2���ش��������⡣
6Cu+SO2���ش��������⡣
��1��Cu+�ļ۵��ӹ����ʾʽΪ__________________��Cu2O��Cu2S�Ƚϣ��۵�ϸߵ���_______��ԭ��Ϊ_____________________________________��
��2��SO2��SO3�ļ�����ȣ����Ǹ������____________������Һ̬SO3��ȴ��289.8Kʱ���̵õ�һ������״�����ṹ�Ĺ��壬��ṹ����ͼ1��ʾ���˹�̬SO3��Sԭ�ӵ��ӻ����������_______���ýṹ��S��O���������࣬һ�����Լ140pm����һ�����ԼΪ160pm���϶̵ļ�Ϊ_________����ͼ����ĸ����
��3�����ӻ�����CaC2��һ�־���ṹ����ͼ2��ʾ��д�������ʵĵ���ʽ_____���Ӹ����ӿ�������____________�ѻ���һ���������е�����ƽ����______����
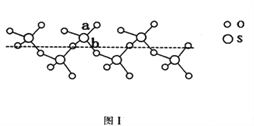
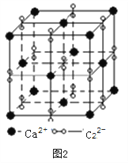

��4����������̼�ܽ����á�Fe���γɵ�һ�ּ�϶�����壬���ԣ��侧������ͼ3��ʾ��������ʵĻ�ѧʽΪ________���������ܶ�Ϊdg/cm3���������������̼ԭ�ӵľ���Ϊ____________________ pm���������ӵ�������ֵ��NA��ʾ��д������ʽ���ɣ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com