
ʵ�����Ʊ�����ͪ�Ļ�ѧ����ʽΪ��
�Ʊ������л���CH3COOH��AlCl3�D��CH3COOAlCl2��HCl���ȸ���Ӧ��
��Ҫʵ��װ�úͲ������£�
(��)�ϳɣ�������ƿ�м���20g��ˮ���Ȼ�����30mL
��ˮ����Ϊ���ⷴӦҺ���¹��죬�߽���������μ�6mL
��������10mL��ˮ���Ļ��Һ�����Ƶμ����ʣ�ʹ��ӦҺ
�����������μ���Ϻ���Ȼ���1Сʱ��
(��)�������ᴿ���ٱ߽���������μ�һ����Ũ�������ˮ���Һ������õ��л����ˮ���ñ���ȡ����Һ�۽��٢������л���ϲ���ϴ�ӡ������ȥ�����õ�����ͪ�ֲ�Ʒ������ֲ�Ʒ�õ�����ͪ
�ش��������⣺(1)����a�����ƣ�________��װ��b�����ã�________��
(2)�ϳɹ�����Ҫ����ˮ������������_______________________��
(3)�����������ͱ��Ļ��Һһ���Ե�������ƿ�����ܵ���________��
| A����Ӧ̫���� | B��Һ��̫������� | C����Ӧ�仺�� | D������������ |

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CuSO4��Һ��K2C2O4��Һ��Ӧ���õ�һ����ɫ�ᾧˮ���ᄃ�塣ͨ������ʵ��ȷ���þ������ɣ�
�ٳ�ȡ0.1680g���壬���������H2SO4��Һ��ʹ��Ʒ�ܽ���������ˮ�����Ƚ��У���0.02000mol��L-1KMnO4��Һ�ζ����յ㣨��Һ��Ϊdz�Ϻ�ɫ��������20.00mL��
�ڽ��Ž���Һ��ּ��ȣ�ʹdz�Ϻ�ɫ��Ϊ��ɫ����ʱMnO��4ת��ΪMn2+���ͷų�O2��
����ȴ�����2g KI���壨������������Na2CO3����Һ��Ϊ��ɫ�����ɳ�����
����0.05000mol��L-1Na2S2O3��Һ�ζ������յ��ָʾ�����ζ����յ㣬����10.00mL��
��֪��2MnO��4+5H2C2O4+6H+==2Mn2++10CO2��+8H2O
2Cu2++4I��=2CuI��+I2
2Na2S2O3+I2=2NaI+Na2S4O6
��1��������з�����Ӧ�����ӷ���ʽΪ ��
��2��������м����ָʾ��Ϊ ��
��3��ͨ������д����ɫ����Ļ�ѧʽ��д��������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о�С�������KMnO4�ⶨFeSO4�ĺ�����
(1)��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ200 mL������ʱ��Ҫ����������ƽ��ҩ���⣬����Ҫ�������Уߣߣߣߣߡ��ߣߣߣߣߡ��ߣߣߣߣߡ��ߣߣߣߣߡ��ߣߣߣߣߡ�
����KMnO4(�ữ)�ζ�ʱ����������������Һ���ڣߣߣߣߣ�(������)�У����������Һ����______(������)�У��ζ��յ�ʱ��Һ����ɫΪ�ߣߣߣߣ�ɫ��
(2)��һ�о�С������������²���������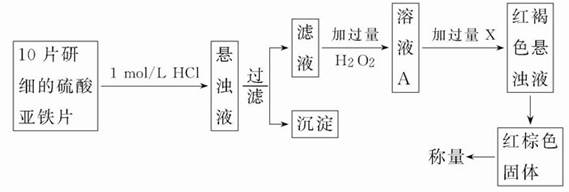
�ٹ���ʱ�õ��IJ��������Уߣߣߣߣߣߣߣߣߣߣߣߣߣߣߣߡ�
�ڴӺ��ɫ������Һ�����ij����������������Ļ��������Уߣߣߣߣ�(��������˳����д)��
A���ˣ�Bϴ�ӣ�C��ȡ��D��Һ��E��ȴ��F����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
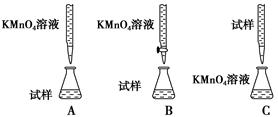
������������(FeSO4��7H2O)��ҽҩ������Ѫ����Ϊ�ⶨ��Ѫ������Ԫ�صĺ�����ij��ѧ��ȤС�����������ʵ�鷽����
����һ���ζ�����������KMnO4��Һ�ζ��ⶨ��Ԫ�صĺ�����
��Ӧԭ����5Fe2����MnO4����8H��===5Fe3����Mn2����4H2O
(1)ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250 mL������ʱ��Ҫ����������ƽ�����������ձ�����ͷ�ι��⣬����________(����������)��
(2)����ʵ����KMnO4��Һ��Ҫ�ữ�������ữ������________��
| A��ϡ���� | B��Ũ���� | C��ϡ���� | D��ϡ���� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
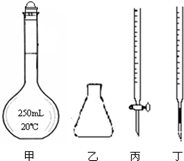
���������һ����Ҫ�Ļ�ѧ�Լ�������Һ�����ȶ��������������»�ֽ����ɶ������̺������������Ի���������Һ�зֽ��ٶȺ���������ֽ��ٶȼӿ졣
��1�����������¸��������Һ�ֽ�����ӷ���ʽ�� ��
��2������ƽ���������Һ�������Na2C2O4��Һ�����������·�Ӧ�����ӷ���ʽ��
______MnO4�� +______C2O42��+______H+=______Mn2++______CO2��+____________
��3��ijѧϰС��Ϊ��̽�����������Һ�Ͳ�������Һ�ķ�Ӧ���̣������������Һ��εص���һ����������Բ�������Һ�У��¶���ͬ����������ʱ������¼���������±���
������������Һ�Ĵ���ÿ����Һ�������ͬ�� | ���������Һ��ɫ��ȥ��ʱ�� |
| �ȵ����1�� | 1min |
| ��ɫ���ٵ����2�� | 15s |
| ��ɫ���ٵ����3�� | 3s |
| ��ɫ���ٵ����4�� | 1s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij������ˮ�к�����̬��(�������ȷ���Cl2)��ͨ������ʵ��ⶨ��Ũ�ȣ�
��ȡ��ˮ��10.0mL����ƿ������10.0 mL KI��Һ(����)������ָʾ��2��3�Ρ�
��ȡһ�ζ�������������ˮ������ˮϴ����Ȼ���ע��0.01 mol��L��1 ��Na2S2O3��Һ������Һ�棬���¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2 + 2Na2S2O3 =" 2NaI" + Na2S4O6 (Na2S4O6��ҺΪ��ɫ)���Իش��������⣺
��1������ټ����ָʾ���� ��
��2������ٷ�Ӧ�����ӷ���ʽ�� ��
��3�����ۢ۵�����Һ�� ɫ��Ϊ ɫ��30s���ٱ仯�����յ㣬����ȥNa2S2O3��Һ20.00mL�����ˮ��C12�����ʵ���Ũ��Ϊ ��
��4����������ʵ����������ᵼ������õ�Cl2�����ʵ���Ũ�Ȼ��ʵ��Ũ�� (�ƫ����ƫС������ȡ�)��
��5������Na2S2O3��Һ��������淶��û��ƽ�ӣ��ζ�ǰ���ӣ��ζ����ָ��ӣ����ᵼ������õ�Cl2�����ʵ���Ũ�Ȼ��ʵ��Ũ�� (�ƫ����ƫС������ȡ�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
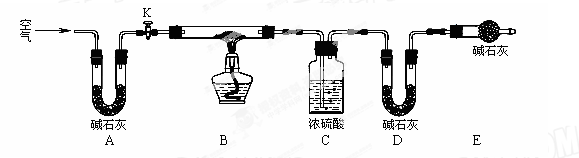
(15��)��ʽ̼��ͭ�ijɷ��ж��֣��仯ѧʽһ��ɱ�ʾΪxCu(OH)2��yCuCO3��
(1)��ȸʯ����ɫ����һ������ı�ʯ������Ҫ�ɷ���Cu(OH)2��CuCO3��ij��ȤС��Ϊ̽����ȡ��ȸʯ����ѷ�Ӧ���������������ʵ�飺
ʵ��1����2.0mL 0.50 mol��L�C1��Cu(NO3)2��Һ��2.0mL 0.50 mol��L�C1��NaOH��Һ��0.25 mol��L�C1��Na2CO3��Һ��������ʾ�����ϡ�
ʵ��2�������ʱ����Ļ�����ڱ�����ʾ�¶��·�Ӧ��
ʵ���¼���£�
���� ����
| ��� | V (Na2CO3)/mL | ������� | | ��� | ��Ӧ�¶�/�� | ������� |
| 1 | 2.8 | �ࡢ��ɫ | | 1 | 40 | �ࡢ��ɫ |
| 2 | 2.4 | �ࡢ��ɫ | | 2 | 60 | �١�dz��ɫ |
| 3 | 2.0 | �϶ࡢ��ɫ | | 3 | 75 | �϶ࡢ��ɫ |
| 4 | 1.6 | ���١���ɫ | | 4 | 80 | �϶ࡢ��ɫ(������ɫ) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��������ƣ�Na2S2O3����������һϵ�з�Ӧ�Ƶã�
��Na2CO3+SO2 =Na2SO3+CO2
��Na2S+SO2+H2O=Na2SO3+H2S
��2H2S+SO2=3S��+2H2O
��Na2SO3 + S  Na2S2O3��
Na2S2O3��
��������Һ����������ΪNa2S2O3?5H2O��Na2S2O3��5H2O��40��45���ۻ���48��ֽ⣻
Na2S2O3������ˮ���������Ҵ�����ˮ���й����ʵ��ܽ��������ͼ��ʾ��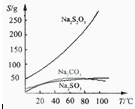
���ְ����·����Ʊ�Na2S2O3��5H2O��
�����ƺ�̼���ư���ӦҪ�����һ������������ƿ�У�ע��150mL����ˮʹ���ܽ⣬�ڷ�Һ©���У�ע��Ũ���ᣬ��װ��2�м����������ƹ��壬������ͼ��װ��װ�á�
���ʣ�����2������Ϊ ��
װ��6�пɷ��� ��
| A��BaCl2��Һ | B��ŨH2SO4 |
| C������KMnO4��Һ | D��NaOH��Һ |

 6I-+Cr2O72-+14H+=3I2+2Cr3++7H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ�� I2+2S2O32-=2I-+S4O62-���ζ��յ������Ϊ ��
6I-+Cr2O72-+14H+=3I2+2Cr3++7H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������Ӧ�� I2+2S2O32-=2I-+S4O62-���ζ��յ������Ϊ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ᴿ�������ʣ������ڵ����������ʣ�����ѡ�õij����Լ��ͷ��뷽������ȷ����(����)
| | ���ᴿ������ | �����Լ� | ���뷽�� |
| A | �������������ᣩ | CCl4 | ��ȡ����Һ |
| B | ����(��ϩ) | ����KMnO4��Һ | ϴ�� |
| C | �屽���壩 | ����������Һ | ��Һ |
| D | �������ӣ� | Ũ��ˮ | ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com