
����Ŀ���ݻ��ɱ���ܱ������У���Ӧ2SO2(g)ʮO2(g) ![]() 2SO3(g)��һ�������´ﵽƽ�⣬���������գ�
2SO3(g)��һ�������´ﵽƽ�⣬���������գ�
��1����ҵ�ϸ÷�Ӧ����________�����豸���ƣ����еģ����õĴ�����________��
��2��������������ʱ�������¶ȣ�ƽ��������Ӧ�����ƶ���������ӦΪ_______��ѡ����ȡ������ȡ�������ͼΪ��Ӧ���ʣ�������ʱ�䣨t���Ĺ�ϵ���ж���t1ʱ�����߷����仯��ԭ����__________��ѡ���ţ���

a.����O2��Ũ��
b. �����������
c. �������
d. �����¶�
��3���ı�������ƽ��������SO3�İٷֺ���_______��ѡ�������С�������䡱����
��4����ҵ���ð�ˮ������SO2β���������γɻ���(NH4)2 SO4����(NH4)2 SO4��������ˮ����Һ��_______�ԣ�������____________________�������ӷ���ʽ��ʾ��������Һ��ϵ�д��ڶ����غ㣬����д������һ���غ��ϵ(������Ũ�ȱ�ʾ) _______________________��
���𰸡� �Ӵ��� ����ý ���� C ���� �� NH4+ + H2O ![]() NH3��H2O + H+ ����غ㣺[NH4+]+[H+]=2[SO42-] + [OH-]�������غ㣺2[SO42-]=[NH4+]+[NH3��H2O]�������غ㣺[H+]=[NH3��H2O] + [OH-]
NH3��H2O + H+ ����غ㣺[NH4+]+[H+]=2[SO42-] + [OH-]�������غ㣺2[SO42-]=[NH4+]+[NH3��H2O]�������غ㣺[H+]=[NH3��H2O] + [OH-]
��������������Ҫ����ƽ����ƶ���
1����ҵ�ϸ÷�Ӧ���ڽӴ��ҽ��еģ����õĴ���������ý��
��2��������������ʱ�������¶ȣ�ƽ��������Ӧ�����ƶ���������Ӧ���ȡ�
t1ʱ�������淴Ӧ����ͬ�ȳ̶��������߷����仯��ԭ����c��
a.����Ӧ��Ũ��ʹ����Ӧ���������淴Ӧ���ʲ��䣬ƽ�����ƣ���a���������⣻b. ���������������ѹ��ƽ�����ƣ���b���������⣻c. �����������Ӧ���ʺ��淴Ӧ����ͬ�ȳ̶�����ƽ�ⲻ�ƶ�����c�������⣻d. �����¶������淴Ӧ��������̶Ȳ�ͬ��ƽ�ⷢ���ƶ�����d���������⡣��ѡc��
��3���ı�������ƽ�ⲻ�ƶ���ƽ��������SO3�İٷֺ������䡣
��4����(NH4)2 SO4��������ˮ����Һ�����ԣ�������![]() +H2O
+H2O![]() NH3 H2O+H+������Һ��ϵ�д��ڶ����غ㣬����һ���غ��ϵ(������Ũ�ȱ�ʾ)������غ㣺[NH4+]+[H+]=2[SO42-] + [OH-]�������غ㣺2[SO42-]=[NH4+]+[NH3��H2O]�������غ㣺[H+]=[NH3��H2O] + [OH-]
NH3 H2O+H+������Һ��ϵ�д��ڶ����غ㣬����һ���غ��ϵ(������Ũ�ȱ�ʾ)������غ㣺[NH4+]+[H+]=2[SO42-] + [OH-]�������غ㣺2[SO42-]=[NH4+]+[NH3��H2O]�������غ㣺[H+]=[NH3��H2O] + [OH-]


 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС��̽��Ԫ�������ɣ�����Ԫ�طǽ��������Ӧ��ۺ�����֮��Ĺ�ϵ���������ͼװ����һ�������N��C��Si�ķǽ�����ǿ���Ƚϵ��о����Ҹ����û���Ӧ�Ĺ��ɣ�������ͼװ�����OԪ�صķǽ����Ա�Sǿ���о����ش�
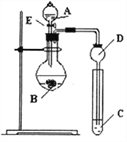
��1��ͼ��Aװ��������______________��
��2������������������ѡ����ͬѧ��Ƶ�ʵ�����õ����ʣ�
��ϡHNO3��Һ ��ϡ���� ��̼��� ��Na2SiO3 ��Һ ��SiO2
�Լ�A��C�ֱ�Ϊ_________������ţ����Թ��з�����Ӧ�����ӷ���ʽΪ_______________.��ͬѧ��Ϊ��ʵ�鲻��˵��N��C��S�ķǽ�����ǿ��������Ϊԭ����_________________
��3����ͬѧ��Ƶ�ʵ�����õ��Լ�AΪ________�����C�Լ�Ϊ�����ˮ��Һ�����Թ��п��Թ۲쵽������Ϊ_______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. HNO3��Ħ������Ϊ63g
B. ���¡���ѹ�£�32��O2����2NA��O
C. ��״���£�1molH2O�����ԼΪ22.4L
D. 1L1mol/LCH3COONa��Һ��c��CH3COO����=1mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�������ȷ���ǣ� ��
A.����ʱ�ò���������©�����Һ��
B.���Ⱥ��������������ǯ��ȡ
C.������ˮ������ˮʵ���У����¶ȼ�ˮ����嵽����ƿ����ˮ��
D.�ӵ�ˮ����ȡ���ʵ�ʱ��������ˮ�Ҵ�����CCl4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���Է�Һ�к���Fe2+��Cu2+��Ba2+���ֽ������ӣ���ͬѧ��������з����Է�Һ���д����������Լ����Թ��������Ի��ս���������������
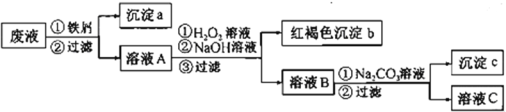
��ش�
��1������a�к��еĵ����ǣ�д��ѧʽ��_______��
��2����ҺA��H2O2��Һ�����������·�Ӧ�����ӷ���ʽ��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ú�����ɵõ���̿��ú���͡��ְ�ˮ�ͽ�¯���ȡ���̿��ͨ������;����ȡ������ϩ�Ȼ�����Ʒ��

���������գ�
��1��������Ȳת��ΪHC��C��CH��CH2�ķ�Ӧ������___________________��
��2��HC��C��CH��CH2������������![]() �Ĺ�ϵ��______________________��
�Ĺ�ϵ��______________________��
��3�����ֱ���ȫȼ�յ�������HC��C��CH��CH2����Ȳ��������������____________________ ��ѡ�ǰ�߶ࡱ�����߶ࡱ��һ���ࡱ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���𡢵���þ������ͭ���ڹ�ҵ�����ж��й㷺����;��
��1��Cu+��̬��������Ų�ʽΪ___________��
��2������Mg��Al�е�һ�����ܽϴ����_________��
��3��������CH3COO[Cu(NH3)3��CO]����Cu+�γ������ӵ�����Ϊ______(�ѧʽ)��
��4��NH4NO3��Nԭ���ӻ��������Ϊ_______��C��N��O����Ԫ�صĵ縺���ɴ�С��˳��Ϊ________�����ݵȵ���ԭ����CO���ӵĽṹʽΪ________��
��5��1molCO(NH2)2��H2O2(����������)�к��е���������ĿΪ_________��
��6�����������������ɻ���У��侧����ͼ��ʾ�����ھ��������ϵ�ԭ�ӵ���λ��Ϊ_____����������������ܶ�Ϊ��g/cm3�������ӵ�������ֵΪNA,��������������Nԭ��֮��ľ���Ϊ_______cm��
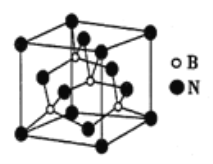
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����⾫��������������г�����һ������Sn��,������Ag��Bi��Cu�Ƚ����������ռ�ֵ��ij��ұ�������ô�ʪ�����մ�����������ȡAg��Bi��Cu,�������£�

��֪����BiCl3+H2O![]() BiOCl+2HCl
BiOCl+2HCl
��SnO2������ϡ��ͼ���Һ
(1)���������ڡ�����������ǰ����鴦����Ŀ����__________________________��
(2)����������"ʱCu������Ӧ�����ӷ���ʽΪ______________________��
(3)����1����Ҫ�ɷ�ΪAgCl��SnO2,��������ʱ������Ӧ�Ļ�ѧ����ʽΪ______________��
(4)ˮ����(N2H4��H2O)��_________����(���ԭ����������)����֪N2H4Ϊ��Ԫ�����ˮ�еĵ����백���ƣ����һ������ķ���ʽΪ________________________��
(5)�������ұ��ʱ��_________����������_________��Һ�����Һ��
(6)����ͭ�顱ʱӦ����Һ��pH����0.5��1.0֮�䣬Ŀ����____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ȡ��Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺

��֪������з�Ӧ�Ļ�ѧ����ʽ��Cl2��2KI===2KCl��I2������д���пհף�
(1)����۵�ʵ�����������________�������ʵ�����������________��
(2)����ݵIJ���������________�����ձ��⣬�������õ��IJ���������________��
(3)�ӵ�ˮ��Һ����ȡ�⣬������ѡ������__________(����ĸ)������Ϊ��ȡ����
a���ƾ�������b����(�ܶȱ�ˮС)������c���Ȼ�����Һ d.����
(4)���¹�����ȡ��Һ�����������У�����ȷ����______��
A��ˮ��Һ�м������ѣ�ת������Һ©���У����ϲ���������ͼ������
![]()
B�����κ����Һ©���ϿڵIJ���������
C�����������������ֳַ�Һ©�����ô�Һ��ֲ�
D����Һʱ�����Ƚ��Ͽڲ������������ϵİ��۶�©�����ϵ�С�ף��ٴ��������²�Һ��ȫ������ʱ���ٴ��Ͽڵ����ϲ�Һ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com