��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ�CH
3OCH
3����
��ش��������⣺
��1��ú�����������в������к�����H
2S��Na
2CO
3��Һ���գ�����������ʽ�Σ��÷�Ӧ�Ļ�ѧ����ʽΪ��
Na2CO3+H2S�TNaHCO3+NaHS
Na2CO3+H2S�TNaHCO3+NaHS
��2������ˮú���ϳɶ����ѵ�������Ӧ���£�
��2H
2��g��+CO��g��?CH
3OH��g����H=-90.8kJ?mol
-1��2CH
3OH��g��?CH
3OCH
3��g��+H
2O��g����H=-23.5kJ?mol
-1��CO��g��+H
2O��g��?CO
2��g��+H
2��g����H=-41.3kJ?mol
-1�ܷ�Ӧ��3H
2��g��+3CO��g��?CH
3OCH
3��g��+CO
2��g���ġ�H=
-246.4kJ?mol-1
-246.4kJ?mol-1
һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�ǣ�
a��c��e
a��c��e
������ĸ���ţ���
a��ѹ����� b��������� c������CO
2��Ũ��
d������CO��Ũ�� e������������ѣ�CH
3OCH
3��
��3����֪��Ӧ�ڣ�2CH
3OH��g��?CH
3OCH
3��g��+H
2O��g����H=-23.5kJ?mol
-1ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH
3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
| ���� |
CH3OH |
CH3OCH3 |
H2O |
| Ũ�ȣ�mol?L-1�� |
0.40 |
0.6 |
0.6 |
�ٱȽϴ�ʱ�����淴Ӧ���ʵĴ�С�Ƚϣ�v
����
��
v
�� �����������������=������
�ڸ÷�Ӧ��ƽ�ⳣ���ı���ʽΪK=
| c(CH3OCH3)c(H2O) |
| c2(CH3OH) |
| c(CH3OCH3)c(H2O) |
| c2(CH3OH) |
�¶����ߣ��÷�Ӧ��ƽ�ⳣ��K
��С
��С
�����������С�����䡱����




 ��2012?�������ģ����������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ������H2��CO2�ϳɶ����ѵķ�Ӧ���£�6H2��g��+2CO2��g��?CH3OCH3��g��+3H2O��g��
��2012?�������ģ����������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ������H2��CO2�ϳɶ����ѵķ�Ӧ���£�6H2��g��+2CO2��g��?CH3OCH3��g��+3H2O��g��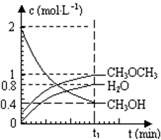 ��֪��������һ����Ҫ�����ȼ�ϣ�����ͨ��CH3OH���Ӽ���ˮ�Ƶã�2CH3OH��g��?CH3OCH3��g��+H2O��g����H=23.5kJ?mol-1����T1�棬�����ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ����ش��������⣺
��֪��������һ����Ҫ�����ȼ�ϣ�����ͨ��CH3OH���Ӽ���ˮ�Ƶã�2CH3OH��g��?CH3OCH3��g��+H2O��g����H=23.5kJ?mol-1����T1�棬�����ܱ������н�������ƽ�⣬��ϵ�и����Ũ����ʱ��仯��ͼ��ʾ����ش��������⣺