
����Ŀ��úȼ���ŷŵ���������SO2��NOx������NaClO2��Һ��Ϊ���ռ���ͬʱ�����������������������������գ�
��1���������������������漰���Ļ�ѧ�������Ԫ���У����ڵ�������Ԫ�ص���___��д��N�ĺ�������Ų�ʽ___��
��2����֪SO2���ӵĿռ乹��Ϊ�����Σ���SO2Ϊ___��ѡ���������������Ǽ����������ӡ�
��3��������SO2��NOx������ͨ��ʢ��NaClO2��Һ�ķ�Ӧ���У���Ӧһ��ʱ�ʺ����Һ������Ũ�ȵ��й��������£��������Ӻ��Բ��ƣ���
���� | Na+ | SO42- | NO3- | OH- | Cl- |
Ũ��/��mol��L-1�� | 5.5��10-3 | 8.5��10-4 | y | 2.0��10-4 | 3.4��10-3 |
�ٷ�Ӧ����ҺpH___7������y=___mol��L-1��
��д��NaClO2��Һ����SO2�����ӷ���ʽ___��
��4�������е�SO2���ɲ��ð��������ȥ���䷴Ӧԭ��������ͼ��ʾ��
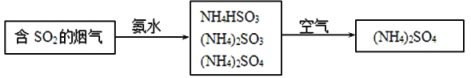
��д��SO2����ˮ��Ӧ����NH4HSO3�Ļ�ѧ����ʽ___��
��(NH4)2SO4��Һ��Ũ������������___��
���𰸡�Na��S��Cl 1s22s22p3 ���� �� 2.0��10-4mol��L-1 ClO2-+2SO2+4OH-��2SO42-+Cl-+2H2O SO2+NH3��H2O��NH4HSO3 NH4+
��������
��1��Nԭ�Ӻ�����7�����ӣ������������ԭ���Ų���
��2��SO2���ӵĿռ乹��Ϊ�����Σ�����������ɵ����IJ��غϣ�
��3���ٸ��ݷ�Ӧ����Һ�������ӵ�Ũ���ж�pH�����ݵ���ؼ���yֵ��
��SO2��NaClO2��Һ����ΪSO42-��NaClO2����ԭΪCl-��
��4����SO2����ˮ1:1��Ӧ����NH4HSO3��
��(NH4)2SO4��ǿ����ʣ���ȫ���룬 c(NH4+)�ӽ� c(SO42-)�Ķ�����
��1������Ԫ�����ڱ������ڵ�������Ԫ�ص���Na��S��Cl��Nԭ�Ӻ�����7�����ӣ���������Ų�ʽ��1s22s22p3��
��2��SO2���ӵĿռ乹��Ϊ�����Σ�����������ɵ����IJ��غϣ�SO2Ϊ���Է��ӣ�
��3���ٷ�Ӧ����Һ�����������ӵ�Ũ����2.0��10-4��������Ũ����5.0��10-11��������Ũ��С�����������ӵ�Ũ�ȣ�������Һ�ʼ��ԣ�pH��7�����ݵ���غ�5.5��10-3+5.0��10-11=(8.5��10-4)��2+y+2.0��10-4+3.4��10-3�����y=2.0��10-4mol��L-1��
��SO2��NaClO2��Һ����ΪSO42-��NaClO2����ԭΪCl-����Ӧ�����ӷ���ʽ��ClO2-+2SO2+4OH-��2SO42-+Cl-+2H2O��
��4����SO2����ˮ1:1��Ӧ����NH4HSO3����Ӧ�Ļ�ѧ����ʽ��SO2+NH3��H2O��NH4HSO3��
��(NH4)2SO4��ǿ����ʣ���ȫ���룬 c(NH4+)�ӽ� c(SO42-)�Ķ���������Ũ������������NH4+��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ľ�ѩ����ͨ���������㣬���д���أ�CH3COOK������ѩЧ����õ���ѩ�������й��ڴ���ػ�����˵����ȷ���ǣ� ��
A.1molCH3COOK������Ϊ98g/mol
B.CH3COOHĦ����������������Է�������
C.һ��CH3COOH������ԼΪ![]() g
g
D.����6.02��1023��̼ԭ�ӵ�CH3COOK�����ʵ�����1mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I��ij��ѧʵ��С��Ϊ��̽����ʵ�����Ʊ�Cl2�Ĺ�������ˮ������HCl�ӷ�������ͬʱ֤��������ijЩ���ʣ�ijͬѧ�������ͼ��ʾ��ʵ��װ�ã�����������CCl4��HCl������CCl4)��
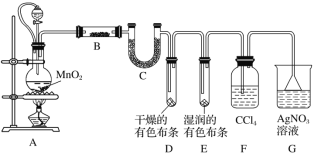
��ش��������⣺
(1)װ��A��������Ƥ�ܵ�Ŀ����_____________________________________��
(2)װ��A�з�����Ӧ�����ӷ���ʽΪ_________________________________��װ��B��ʢ�ŵ��Լ���___________��
(3)װ��D��E�г��ֵIJ�ͬ����˵����������_________________________��
(4)��ͬѧ����ʵ����Ͻ��Կ��ǣ���Ϊ����F��G����װ��֮���ټ�һ��װ��ʪ��ĵ���KI��ֽ��װ�ã���Ŀ����___________________________________��
��ij�о���ѧϰС�����Ʊ�Ư�ۣ����������װ��A����������ͼ��ʾ�����װ�ã�
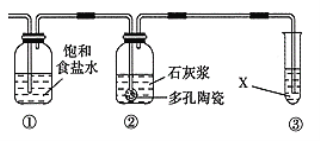
(5)װ�â��з�����Ӧ�Ļ�ѧ����Ϊ___________________________________��
(6)��Ư�۵ķ�Ӧ�Ƿ��ȷ�Ӧ����Ӧ�¶Ƚϸ�ʱ�и���Ӧ�������Ľ���ʵ��װ���Լ��ٸ���Ӧ�����ķ�����___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)��������8��Ԫ�ذ������۵�ߵ͵�˳����ͼ�����������8������___(��Ԫ�ط���)�����е縺��������___����ͼ�е���ţ���
(2)��д����Ԫ��1��ԭ��������8��Ԫ�صĻ�̬ԭ�ӵ����Ų�ʽ___��
(3)Ԫ��7�ĵ��ʾ�����ԭ�ӵĶѻ���ʽ��ͼ����ʾ���侧��������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ��ͼ����ʾ��
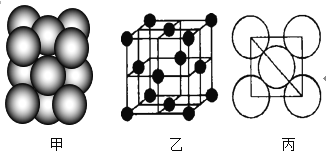
����֪7��ԭ�Ӱ뾶Ϊd cm��NA���������ӵ�������7�����ԭ������ΪM����ش�
�پ�����7ԭ�ӵ���λ��Ϊ___��һ��������7ԭ�ӵ���ĿΪ___��
�ڸþ�����ܶ�Ϊ___ g/cm3(����ĸ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���42��XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӡ�X��Y���γɻ�����X2Y3��ZԪ�ؿ����γɸ�һ�����ӡ���ش��������⣺
(1)XԪ��ԭ�ӻ�̬ʱ�ĵ����Ų�ʽΪ_______________��
(2)YԪ��ԭ�ӵļ۲���ӵĹ����ʾʽΪ_____________��
(3)X��Z���γɻ�����XZ3���û�����Ŀռ乹��Ϊ________��
(4)��֪������X2Y3��ϡ������Һ�пɱ�����п��ԭΪXZ3�����ﻹ��ZnSO4��H2O���÷�Ӧ�Ļ�ѧ����ʽ��___________��
(5)�Ƚ�X���⻯����ͬ��ڶ�����������Ԫ�����γɵ��⻯��е�ߵ�______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������E��������-1��4-��������������һ�������������ϵ�ԭ�ϡ���ϳ�·�����£�

���������գ�
��1��A�к��еĹ�������______��E�ķ���ʽ��______���Լ�a��______��
��2��д����Ӧ���ͣ�B��C______��
��3��C��D��Ӧ����E�Ļ�ѧ����ʽ��______��
��4��C��ͬ���칹�壬��ʹʯ���Լ��Ժ�ɫ��д�������ʵ�һ�ֽṹ��ʽ______��
��5�����һ���Ի�������![]() ��Ϊԭ�ϣ��������Լ���ȡ���ϳ�A�ĺϳ�·�ߡ�
��Ϊԭ�ϣ��������Լ���ȡ���ϳ�A�ĺϳ�·�ߡ�
���ϳ�·�߳��õı�ʾ��ʽΪ��A![]() B����
B����![]() Ŀ����_____________________
Ŀ����_____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽��ij��ɫ���ᾧˮ����X����ɺ����ʣ���Ʋ������ͼʵ�顣��ش�

��1��X�Ļ�ѧʽ��___��
��2���һ�ѧ�����������Ԫ�صĵ������ƣ����������������ҷ�����Ӧ����__����д��Ӧ����ĸ����
A.Mg B.CaCl2 C.NaOH D.K2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�ڢ�![]() Li����ʯī����C60����
Li����ʯī����C60����![]() Mg �� CH3CH2OH ��
Mg �� CH3CH2OH ��![]() C ��
C ��![]() Li �� CH3OCH3 �У�____��Ϊͬλ�أ� ____��Ϊͬ���칹�壻___��Ϊͬ��������(�����)
Li �� CH3OCH3 �У�____��Ϊͬλ�أ� ____��Ϊͬ���칹�壻___��Ϊͬ��������(�����)
(2)���Т�CaCl2 �ڽ��ʯ ��NH4Cl ��Na2SO4 �ݱ� ���������ʣ�������Ҫ��ش�
���ۻ�ʱ����Ҫ�ƻ���ѧ������___________���۵���ߵ���_______��(�����)
��ֻ�������Ӽ���������______�������Է��Ӽ���������ϵ���______��(�����)
(3)д���������ʵĵ���ʽ
��H2O______
��NaOH______
��NH3______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������������������գ�
��1��ͬ��ͬѹ�£�H2(g)+Cl2(g)��2HCl(g)�����պ͵�ȼ��������H����ѧ��������ͬ���ֱ�Ϊ��H1����H2����H1______��H2��
��2����ͬ�����£�2mol��ԭ�������е�����____1mol����������е�������
��3����֪����ʱ���ױȰ����ȶ����Ƚ����з�Ӧ����H�Ĵ�С����H1_____��H2��
��4P(���ף�s) +5O2(g)��2P2O5(s) ��H1����4P(���ף�s)+5O2(g)��2P2O5(s) ��H2��
��4����֪��101 kPaʱ��2C(s) +O2(g)��2CO(g) ��H����221kJ��mol��1����̼��ȼ������ֵ____110.5 kJ��mol��1��
��5����֪��ϡ��Һ�У�H��(aq)+OH��(aq)��H2O(l) ��H����57.3kJ/mol����Ũ������ϡNaOH��Һ��Ӧ����1 molˮ���ų�������________57.3 kJ��
��6�����淴Ӧ��aA(��)+bB(��)![]() cC(��)+dD(��)����H��Q������ͼ�ش�
cC(��)+dD(��)����H��Q������ͼ�ش�

P1______ P2���ڣ�a+b��______��c+d������t1��______ t2�档
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com