
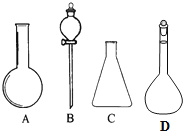
 ��ͼ��ʾA��B��C��D�����������ش��������⣮
��ͼ��ʾA��B��C��D�����������ش��������⣮���� ��1�����л�����ƿ����װ��ʹ��ǰҪ����Ƿ�©ˮ��
��2����������һ�����ʵ���Ũ����Һ����ȷ�������輰����ƿ��ʹ�÷������
��� �⣺��1������ƿ����ƿ������Һ©�����л���ʹ��ǰӦ����Ƿ�©ˮ��
��ѡ����Һ©��������ƿ��
��2��������ƿʹ�ù������ڶ���ʱ��Ҫ������������ˮ������δ�����������ƻᵼ�����ƽ����Ӱ�죬�ʴ���
������ƿΪ����������ֻ���������²ⶨ�������ȷ��������������Һ��Һʱ�ų��������ȣ��ܽ�������������Һ��������Һ�����ȷ����ҺŨ��ƫ�ʴ���
���ڶ��ݲ����и���Һ�棬������Һ���ƫС������C=$\frac{n}{V}$��֪��ҺŨ��ƫ�ߣ�����ȷ��
�ܶ���ʱ��Һ�泬���̶��ߣ��õζ����ƶ�һ����Һ�壬ʹҺ��պ���̶������У����²���������ģ����ʵ����ʵ���ƫС������C=$\frac{n}{V}$��֪��ҺŨ��ƫ�ͣ��ʴ���
��ѡ���ۣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ���Ͳ��������ǽ���ؼ���ע������ƿ�Ĺ��켰ʹ�÷�������Ŀ�ѶȲ���


 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ni���������CO��ת���ʣ�Ni��ת���ʽ��� | |
| B�� | ��С�����ݻ���ƽ�����ƣ���H��С | |
| C�� | ��Ӧ�ﵽƽ�����CO�ٴδﵽƽ��ʱ��CO������������� | |
| D�� | ��4v��[Ni��CO��4]=v����CO��ʱ�������л�������ܶȲ���ʱ������˵����Ӧ�Ѵﻯѧƽ��״̬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
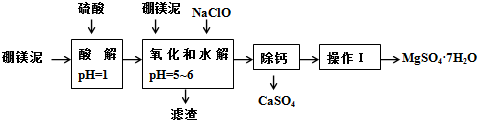
| �������� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Mg��OH��2 | 9.3 | 10.8 |
| Fe��OH��2 | 7.6 | 9.6 |
| Fe��OH��3 | 2.7 | 3.7 |
| Al��OH��3 | 3.7 | 4.7 |
| �¶ȣ��棩 | 40 | 50 | 60 | 70 |
| MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
| CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
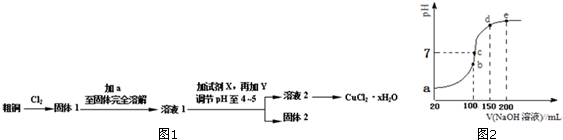
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  �㵹Һ�� | B�� | 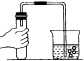 ���װ�õ������� | ||
| C�� | 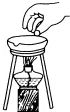 �����ȵ������� | D�� | 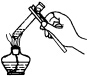 ����Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
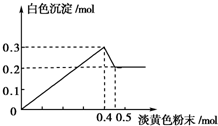 ��һ����Һ����֪���п��ܺ���Fe3+��Mg2+��Cu2+��Al3+��NH4+������һ�ֵ���ɫ��ĩ����ʱ�������д̼�����ζ�Ļ������ų���ͬʱ���ɰ�ɫ������������0.4mol����ɫ��ĩʱ����������0.3mol���������뵭��ɫ��ĩʱ�������̼�����ζ�����壬�Ҽ��뵭��ɫ��ĩʱ������ɫ������������ͼ��ʾ������֪��NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O����
��һ����Һ����֪���п��ܺ���Fe3+��Mg2+��Cu2+��Al3+��NH4+������һ�ֵ���ɫ��ĩ����ʱ�������д̼�����ζ�Ļ������ų���ͬʱ���ɰ�ɫ������������0.4mol����ɫ��ĩʱ����������0.3mol���������뵭��ɫ��ĩʱ�������̼�����ζ�����壬�Ҽ��뵭��ɫ��ĩʱ������ɫ������������ͼ��ʾ������֪��NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ͷ��NaOH��Һ�У�2Al+2OH-+H2O�T2AlO2-+2H2�� | |
| B�� | ʯӢ���ռӦ��SiO2+2OH-�TSiO32-+H2O | |
| C�� | �Ȼ�����Һ�м�������İ�ˮ��Al3++4NH3•H2O�TAlO2-+4NH4++2H2O | |
| D�� | ��С�մ���Һ�м������Ba��OH��2��2HCO3-+Ba2++2OH-�TBaCO3��+2H2O+CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
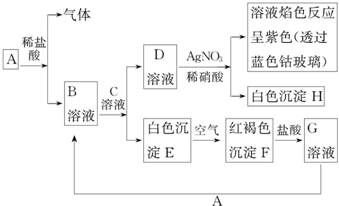
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com