
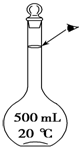
 ʵ��������500mL 0.5mol•L-1��NaCl��Һ�������²������裺
ʵ��������500mL 0.5mol•L-1��NaCl��Һ�������²������裺���� ��1����������500mL 0.5mol/L��NaCl��Һ�IJ���Ը�������������
��2�������������ֱ�Ӽ�ˮ�������Ҫ���ý�ͷ�ι�Ϊ���ݲ�����
��3������ʱ���ӿ̶��ߣ�����������Һ��Һ�����ƫС��Ũ��ƫ�ߣ�
��4������C=$\frac{n}{V}$��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
��5����ʵ������м�����ˮʱ���������˿̶��ߣ�ʵ��ʧ���Ҳ��ܲ��ȣ������������ƣ�
��6����������һ�����ʵ���Ũ����Һ��һ�㲽�裬ѡ����ʵ�������
��� �⣺��1������500mL 0.5mol/L��NaCl��Һ�IJ���Ϊ�����㡢�������ܽ⡢ת�ơ�ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ������˳��Ϊ���٢ڢܢۢݣ�
�ʴ�Ϊ���٢ڢܢۢݣ�
��2������ƿ���п̶��ߣ���Һ��Һ��ײ���̶������У���ʾ�ﵽ�������������������ֱ�Ӽ�ˮ�������Ҫ���ý�ͷ�ι�Ϊ���ݲ�����
�ʴ�Ϊ�����ݣ�
��3������ʱ���ӿ̶��ߣ�����������Һ��Һ�����ƫС��Ũ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��4��δϴ���ܽ�NaCl���ձ��Ͳ��������ᵼ�����ʵ���ʧ������ҺŨ��ƫ�ͣ�������ˮʱ���������˿̶ȣ�������Һ���ƫ����Ũ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ�ƫ�ͣ�
��5����ʵ������м�����ˮʱ���������˿̶��ߣ�ʵ��ʧ���Ҳ��ܲ��ȣ������������ƣ�
�ʴ�Ϊ���������ƣ�
��6�����Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ò�������������ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ��������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ���Ͳ�����貣������
�ʴ�Ϊ����������
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ��ע��c=$\frac{n}{V}$����Ӧ�ã���Ŀ�ѶȲ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Ӱ뾶��Y��X��W | B�� | ���ʵ������ԣ�W��Y��Z | ||
| C�� | ������ZW2����ǿ��ԭ�� | D�� | ������Y2Z2��ֻ�����ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ��һ�ϵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
��ʵ���Ҵ�����ˮ��ȡ����ˮ��ʵ���У�����˵���������
A����ƿ��Ҫ�������Ƭ��ֹ���� 
B���¶ȼƵ�ˮ�������֧�ܿڸ���λ�ã����ܲ���Һ����
C������ˮӦ�����½��ϳ����������������෴
D����ʼ����ʱ��Ӧ���ȼ��ȣ��ٿ�����ˮ��������ϣ�Ӧ���ȹ�����ˮ�ٳ��ƾ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����NaOH������ | B�� | NaOH�ܽ������ת�Ƶ�����ƿ�� | ||
| C�� | ������NaOH��Һ�������ձ��� | D�� | ������ƿ��ˮʱ�۾�һֱ����Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| CH3COOH | H2CO3 | H2S | H3PO4 |
| 1.8��10-5 | K1=4.3��10-7 K2=5.6��10-11 | K1=9.1��10-8 K2=1.1����10-12 | K1=7.5��10-3 K2=6.2��10-8 K3=2.2��10-15 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��pH=4������ʹ���ϡ�ͳ�pH=5����Һ��������������pH��� | |
| B�� | ����ʹ��ᶼ������Ӧ��������Ũ�����ᷴӦ��ȡ | |
| C�� | ��ͬpH������ʹ�����Һ�зֱ������Ӧ�����ι��壬�����pH��� | |
| D�� | ��ͬpH������ʹ���ֱ��п��Ӧʱ��������������ʼ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��ѧʽ | AgCl | Ag2CrO4 | CH3COOH | HClO | H2CO3 |
| Ksp��Ka | Ksp=1.8��10-10 | Ksp=2.0��10-12 | Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.1��10-7 Ka2=5.6��10-11 |
| A�� | ��ͬŨ��CH3COONa��NaClO�Ļ��Һ�У�������Ũ�ȵĴ�С��ϵ��c��Na+����c��ClO-����c��CH3COO-����c��OH-����c��H+�� | |
| B�� | ̼������Һ�еμ�������ˮ�����ӷ���ʽH2O+2CO32-+Cl2=2HCO3-+Cl-+ClO- | |
| C�� | ��0.1mol/LCH3COOH��Һ�еμ�NaOH��Һ��c��CH3COOH����c��CH3COO-��=9��5����ʱ��ҺpH=5 | |
| D�� | ��Ũ�Ⱦ�Ϊ1��10-3mol/L��KCl��K2CrO4���Һ�еμ�1��10-3mol/L��AgNO3��Һ��CrO42-���γɳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
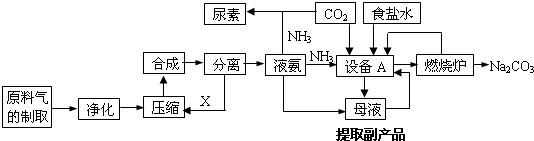
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com